三酶样活性纳米反应器通过气体/饥饿协同作用促进肿瘤铁下垂和轻度光热治疗
IF 9.7
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
轻度光热疗法(mPTT)经常受到热休克蛋白(HSPs)上调的阻碍。为了解决这一限制,我们设计了一个三酶模拟纳米反应器(MPGH),通过葡萄糖氧化酶(GOx)掺杂透明质酸功能化负载mnco的聚多巴胺(PDA)纳米球。MPGH整合了GOx、过氧化物酶(POD)和谷胱甘肽过氧化物酶(GPX)的催化活性。具体来说,GOx消耗葡萄糖诱导饥饿治疗,同时产生H2O2。产生的H2O2促进MnCO分解,释放CO用于气体治疗,Mn2+触发线粒体功能障碍和活性氧(ROS)的产生,从而表现出类似pod的活性。同时,PDA的gpx样活性消耗细胞内谷胱甘肽(GSH),下调谷胱甘肽过氧化物酶4 (GPX4),增强铁下垂。重要的是,co介导的气体治疗和gox介导的饥饿治疗都会破坏细胞能量代谢,降低三磷酸腺苷(ATP)水平,抑制HSP表达,从而显著增强mPTT的疗效。这种破坏能量稳态的协同策略证明了对乳腺癌的有效治疗效果。本文章由计算机程序翻译,如有差异,请以英文原文为准。
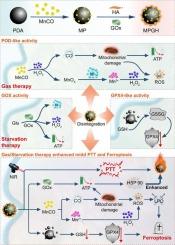
Trienzyme-like activities nanoreactor boosts tumor ferroptosis and mild photothermal therapy via gas/starvation synergy
Mild photothermal therapy (mPTT) is often hindered by the upregulation of heat shock proteins (HSPs). To address this limitation, we designed a trienzyme-mimicking nanoreactor (MPGH) by functionalizing MnCO-loaded polydopamine (PDA) nanospheres with glucose oxidase (GOx)-doped hyaluronic acid. MPGH integrates the catalytic activities of GOx, peroxidase (POD), and glutathione peroxidase (GPX). Specifically, GOx consumes glucose to induce starvation therapy while simultaneously generating H2O2. The produced H2O2 promotes MnCO decomposition, releasing CO for gas therapy and Mn2+ to trigger mitochondrial dysfunction and reactive oxygen species (ROS) generation, thereby exhibiting POD-like activity. Meanwhile, the GPX-like activity of PDA depletes intracellular glutathione (GSH), downregulating glutathione peroxidase 4 (GPX4) to enhance ferroptosis. Importantly, both CO-mediated gas therapy and GOx-mediated starvation therapy disrupt cellular energy metabolism, reducing adenosine triphosphate (ATP) levels and suppressing HSP expression, which markedly enhances the efficacy of mPTT. This synergistic strategy of disrupting energy homeostasis demonstrated potent therapeutic outcomes against breast cancer.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
16.10
自引率
7.10%
发文量
2568
审稿时长
2 months
期刊介绍:
The Journal of Colloid and Interface Science publishes original research findings on the fundamental principles of colloid and interface science, as well as innovative applications in various fields. The criteria for publication include impact, quality, novelty, and originality.
Emphasis:
The journal emphasizes fundamental scientific innovation within the following categories:
A.Colloidal Materials and Nanomaterials
B.Soft Colloidal and Self-Assembly Systems
C.Adsorption, Catalysis, and Electrochemistry
D.Interfacial Processes, Capillarity, and Wetting
E.Biomaterials and Nanomedicine
F.Energy Conversion and Storage, and Environmental Technologies

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


