一种基于超分子铜螯合剂的纳米剂,用于通过铜耗尽和葡萄糖剥夺治疗癌症
IF 9.7
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
鉴于恶性肿瘤对铜的依赖性,铜耗尽疗法已成为一种有前景的抗癌策略。然而,其功效受到肿瘤代谢适应性的阻碍,肿瘤代谢适应性允许肿瘤细胞将其代谢表型转向糖酵解以补偿能量损失。在此,我们开发了一种葡萄糖氧化酶(GOx)集成纳米剂(称为GDP NPs),使用超分子铜螯合剂(称为DP NPs)作为骨架。这种纳米试剂使细胞内铜的消耗和糖酵解底物的剥夺同时发生。超分子螯合剂本身是通过2,2 ' -二聚胺-过官能化柱芳烃的自组装形成的;它消耗铜来放大氧化应激和减少三磷酸腺苷的产生。同时,GOx通过催化葡萄糖分解破坏铜耗尽诱导的代偿性糖酵解途径。与缺铜单药治疗相比,缺铜联合代谢代偿阻断可导致肿瘤细胞能量危机。体内实验结果表明,相对于DP NPs, GDP NPs具有更好的抗癌效果。我们的工作提出了一种基于超分子化学的新策略,可以同时消耗铜和干扰代谢,克服了铜消耗单一疗法因肿瘤代谢适应性而受到的限制。本文章由计算机程序翻译,如有差异,请以英文原文为准。
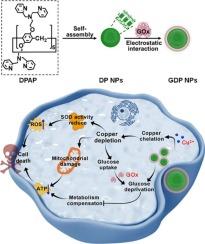
A nanoagent based on a supramolecular copper chelator for cancer therapy via copper depletion and glucose deprivation
Given the copper dependence of malignancies, copper depletion therapy has emerged as a promising anticancer strategy. However, its efficacy is hampered by the metabolic adaptability of tumors, which allows tumor cells switch their metabolic phenotype towards glycolysis to compensate for energy loss. Herein, we developed a glucose oxidase (GOx)-integrated nanoagent (designated as GDP NPs) using a supramolecular copper chelator (denoted as DP NPs) as the backbone. This nanoagent enables the simultaneous intracellular depletion of copper and deprivation of glycolytic substrates. The supramolecular chelator itself is formed via the self-assembly of 2,2′-dipicolylamine-perfunctionalized pillararene; it depletes copper to amplify oxidative stress and reduce adenosine triphosphate production. Concurrently, GOx disrupts the compensatory glycolytic pathway induced by copper depletion through the catalytic breakdown of glucose. Compared with copper depletion monotherapy, the combined effect of copper depletion and metabolic compensation blockade leads to an energy crisis in tumor cells. In vivo experimental results demonstrate that GDP NPs exhibit superior anticancer efficacy relative to DP NPs. Our work presents a novel supramolecular chemistry-based strategy for the concurrent depletion of copper and interference with metabolism, which overcomes the limitation of copper depletion monotherapy due to tumor metabolic adaptability.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
16.10
自引率
7.10%
发文量
2568
审稿时长
2 months
期刊介绍:
The Journal of Colloid and Interface Science publishes original research findings on the fundamental principles of colloid and interface science, as well as innovative applications in various fields. The criteria for publication include impact, quality, novelty, and originality.
Emphasis:
The journal emphasizes fundamental scientific innovation within the following categories:
A.Colloidal Materials and Nanomaterials
B.Soft Colloidal and Self-Assembly Systems
C.Adsorption, Catalysis, and Electrochemistry
D.Interfacial Processes, Capillarity, and Wetting
E.Biomaterials and Nanomedicine
F.Energy Conversion and Storage, and Environmental Technologies

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


