KCl对CeO2-TiO2催化剂同时去除NO和甲苯的影响:来自实验和DFT研究的见解
IF 9.7
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
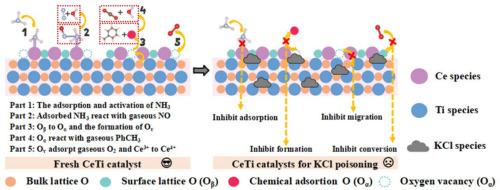
有机固体废弃物或生物质在燃煤锅炉共烧过程中释放的KCl物质不可避免地会危害到脱硝和甲苯氧化催化剂的去除性能和性能。本文研究了KCl对典型CeO2/TiO2催化剂在脱氮和甲苯同时氧化条件下的影响。在400℃时,新鲜CeO2/TiO2催化剂对NOx和甲苯的最佳去除率分别为96.3%和96.4%,而KCl中毒后的去除率分别降至57.10%和15.5%。通过综合表征技术和理论计算,进一步研究了KCl中毒机理。结果表明,在物理上,KCl覆盖了表面活性位点,引起了颗粒聚集,影响了TiO2晶格。化学上,氯化钾降低了氨的吸附能力,降低了表面酸度。同时,由于KCl与CeO和TiO成键,抑制了催化剂上的电子转移,阻碍了活性O/Ce的转化,抑制了氧空位的形成。活性Ce3+的比例降低,导致Ce3+/Ce4+价循环困难。这项工作为富kcl环境中脱氮和甲苯同时氧化的发展奠定了指导意义。本文章由计算机程序翻译,如有差异,请以英文原文为准。
The effect of KCl on the simultaneous removal of NO and toluene on CeO2-TiO2 catalyst: insights from experimental and DFT studies
KCl species released from co-firing of organic solid waste or biomass in coal-fired boilers inevitably jeopardize the removal performance and property of deNOx and toluene oxidation catalysts. Herein, the effect of KCl on the typical CeO2/TiO2 catalyst in the simultaneous deNOx and toluene oxidation scenario was investigated in this paper. The optimal removal efficiencies of NOx and toluene for the fresh CeO2/TiO2 catalyst were 96.3 % and 96.4 % at 400 °C, whereas after KCl poisoning the removal efficiencies decreased to 57.10 % and 15.5 %, respectively. Through comprehensive characterization techniques and theoretical calculations, the KCl poisoning mechanism was further investigated. The findings revealed that physically, KCl covered the surface active sites, caused particle clustering and affected the TiO2 lattice. Chemically, KCl reduced the adsorption capacity of ammonia and decreased the surface acidity. Meanwhile, the electron transfer on the catalyst was also inhibited due to KCl bonding with Ce![]() O and Ti
O and Ti![]() O, hindering the conversion of reactive O/Ce species and inhibiting oxygen vacancy formation. The ratio of active Ce3+ was depressed, leading to difficulty in Ce3+/Ce4+ valence cycling. This work solidifies guidance for the development of simultaneous deNOx and toluene oxidation in rich KCl-containing environments.
O, hindering the conversion of reactive O/Ce species and inhibiting oxygen vacancy formation. The ratio of active Ce3+ was depressed, leading to difficulty in Ce3+/Ce4+ valence cycling. This work solidifies guidance for the development of simultaneous deNOx and toluene oxidation in rich KCl-containing environments.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
16.10
自引率
7.10%
发文量
2568
审稿时长
2 months
期刊介绍:
The Journal of Colloid and Interface Science publishes original research findings on the fundamental principles of colloid and interface science, as well as innovative applications in various fields. The criteria for publication include impact, quality, novelty, and originality.
Emphasis:
The journal emphasizes fundamental scientific innovation within the following categories:
A.Colloidal Materials and Nanomaterials
B.Soft Colloidal and Self-Assembly Systems
C.Adsorption, Catalysis, and Electrochemistry
D.Interfacial Processes, Capillarity, and Wetting
E.Biomaterials and Nanomedicine
F.Energy Conversion and Storage, and Environmental Technologies

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


