催化多组分偶联反应的n -杂环碳银配合物的设计、合成及生物学研究
IF 2.6
3区 化学
Q2 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
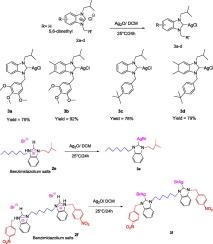
以不同的烷基取代了两个氮原子,合成了高收率的苯并咪唑银盐2a-f。这些盐很容易转化为相应的n -杂环碳(NHC)银(I)配合物3a-f。采用常规波谱方法(1H NMR, 13C{1H} NMR, FTIR)测定了这些配合物的溶液结构,并利用密度泛函理论(DFT)在B3LYP水平上对其分子结构进行了优化。酶抑制和抗氧化实验表明,新的Ag-NHC配合物3a-f的生物活性强烈依赖于NHC取代基的性质。分子对接证实了乙酰胆碱与乙酰胆碱的高结合亲和力,其相互作用发生在外周和催化阴离子位点,而ADMET分析预测了良好的药代动力学和毒性谱。最后,使用基于5,6-二甲基苯并咪唑的nhc -银(I)催化剂,采用温和高效的a (Yılmaz等人,20243)偶联方案,实现了丙炔胺的选择性合成。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Design, synthesis, and biological investigation of N-heterocyclic carbene –silver complexes for catalytic multicomponent coupling reactions
Benzimidazolium silver salts 2a–f, bearing two nitrogen atoms substituted with different alkyl groups, were synthesized in high yields. These salts were readily converted into the corresponding N-heterocyclic carbene (NHC) silver(I) complexes 3a–f. The solution structures of these complexes were determined by mean of conventional spectroscopic methods (1H NMR, 13C{1H} NMR, FTIR) and their molecular structures optimized using density functional theory (DFT) at B3LYP level. Enzyme inhibition and antioxidant assays revealed that the biological activity of the new Ag–NHC complexes 3a–f strongly depend on the nature of the NHC substituents. Molecular docking confirmed high binding affinities toward AChE, with interactions taking place at both the peripheral and catalytic anionic sites, while ADMET analysis predicted favorable pharmacokinetic and toxicity profiles. Finally, a mild and efficient A(Yılmaz et al., 20243)-coupling protocol using 5,6-dimethylbenzimidazole-based NHC–silver(I) catalysts enabled the selective synthesis of propargyl amines.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Polyhedron
化学-晶体学
CiteScore
4.90
自引率
7.70%
发文量
515
审稿时长
2 months
期刊介绍:
Polyhedron publishes original, fundamental, experimental and theoretical work of the highest quality in all the major areas of inorganic chemistry. This includes synthetic chemistry, coordination chemistry, organometallic chemistry, bioinorganic chemistry, and solid-state and materials chemistry.
Papers should be significant pieces of work, and all new compounds must be appropriately characterized. The inclusion of single-crystal X-ray structural data is strongly encouraged, but papers reporting only the X-ray structure determination of a single compound will usually not be considered. Papers on solid-state or materials chemistry will be expected to have a significant molecular chemistry component (such as the synthesis and characterization of the molecular precursors and/or a systematic study of the use of different precursors or reaction conditions) or demonstrate a cutting-edge application (for example inorganic materials for energy applications). Papers dealing only with stability constants are not considered.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


