双金属pt - pd掺杂ZnCo₂O₄尖晶石纳米电催化剂的协同电子调谐和活性位点优化
IF 9.7
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
在深入研究中,我们开发了一系列电催化剂,将铂(Pt)和钯(Pd)掺杂到锌钴酸盐体系中,得到了ZnPtxPdxCo2−2xO4@NF0≤x≤0.08纳米电催化剂。在控制的低浓度(< 8%)下引入贵金属Pt和Pd以优化催化性能。采用原位水热法直接在泡沫镍(NF)上合成了电催化剂。通过XRD、SEM、TEM、HR-TEM、EDX和XPS等综合表征,证实了催化剂的立方尖晶石氧化物结构、形貌和化学组成。优化后的催化剂(x = 0.08)在−10 mA/cm2下的过电位为55 mV, Tafel斜率为23 mV/dec。密度泛函理论(DFT)计算表明,Pt和Pd共掺杂ZnCo2O4通过改变电子结构、降低水解离障碍和促进活性位点间的协同吸附,增强了析氢反应(HER)活性。具体来说,虽然Pt位点表现出强烈的H∗吸附(∆GH∗=−0.522 eV),但这被相邻O位点的近热中性吸附(∆GH∗=−0.106 eV)所抵消,从而产生协同效应,减轻了ZnPtxPdxCo2−2xO4的潜在活性位点中毒。这种互补的相互作用通过平衡催化剂表面的吸附强度来实现持续的氢气生产。Pd和Co的存在进一步促进了这种调节,支持了高效的HER动力学。这些发现表明,双金属掺杂是一种很有前途的优化绿色制氢电催化剂的策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
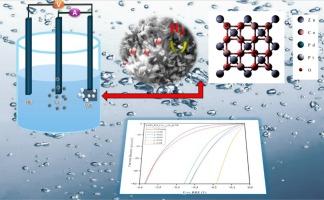
Synergistic electronic tuning and active site optimization in bimetallic Pt-Pd-Doped ZnCo₂O₄ spinel nanoelectrocatalyst for boosted electrocatalytic green hydrogen evolution supported by DFT
In this in-depth study, we developed a series of electrocatalysts by doping platinum (Pt) and palladium (Pd) into the zinc cobaltite system, yielding nanoelectrocatalyst. The noble metals Pt and Pd were introduced in controlled, low concentrations (< 8 %) to optimize the catalytic performance. The electrocatalysts were synthesized directly on nickel foam (NF) using an in situ hydrothermal method. Comprehensive characterization, including XRD, SEM, TEM, HR-TEM, EDX, and XPS, confirmed the cubic spinel oxide structure, morphology, and chemical composition of the catalysts. The optimized catalyst (x = 0.08) exhibited an impressive overpotential of 55 mV at −10 mA/cm2, accompanied by a Tafel slope of 23 mV/dec. Density functional theory (DFT) calculations revealed that co-doping with Pt and Pd enhances hydrogen evolution reaction (HER) activity through modification of the electronic structure, reduction of water dissociation barriers, and facilitation of synergistic adsorption across active sites. Specifically, while Pt sites exhibit strong adsorption ( = −0.522 eV), this is counterbalanced by the nearly thermoneutral adsorption at adjacent O sites ( = −0.106 eV), resulting in a synergistic effect that mitigates potential active site poisoning on . This complementary interaction enables sustained hydrogen production by balancing adsorption strengths across the catalyst surface. The presence of Pd and Co further contributes to this moderation, supporting efficient HER kinetics. These findings establish bimetallic doping as a promising strategy for optimizing electrocatalysts for green hydrogen production.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
16.10
自引率
7.10%
发文量
2568
审稿时长
2 months
期刊介绍:
The Journal of Colloid and Interface Science publishes original research findings on the fundamental principles of colloid and interface science, as well as innovative applications in various fields. The criteria for publication include impact, quality, novelty, and originality.
Emphasis:
The journal emphasizes fundamental scientific innovation within the following categories:
A.Colloidal Materials and Nanomaterials
B.Soft Colloidal and Self-Assembly Systems
C.Adsorption, Catalysis, and Electrochemistry
D.Interfacial Processes, Capillarity, and Wetting
E.Biomaterials and Nanomedicine
F.Energy Conversion and Storage, and Environmental Technologies

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


