近环境条件下动态TiO2载体上的Pt粒子——尺寸、压力和支撑效应
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
已知铂颗粒在可还原氧化物上在高压和高温下形成复杂和高度动态的催化剂体系,通常采用不同于室温和超高真空(UHV)下发现的活性结构。本文利用近环境压力x射线光电子能谱(NAP- xps)、扫描隧道显微镜(STM)和低能离子散射(LEIS)技术,研究了在UHV至0.1 mbar的氧压范围内,金红石TiO2(110)上负载的亚纳米Pt团簇和纳米颗粒的氧化和结构演变。我们的研究结果揭示了Pt纳米颗粒和团簇之间氧化行为和热稳定性的明显差异,这些差异进一步受到载体化学计量和氧压的调节。小的铂团簇即使在室温下也会被氧化,但在高温下0.1毫巴的O2中容易加速烧结。相比之下,在接近化学计量的TiO2上,结晶良好的Pt纳米颗粒表现出较弱的氧化。在还原的、有缺陷的TiO2载体上,Pt很快被在载体再氧化过程中形成的新二氧化钛层深埋。这一过程似乎主要是由支撑物与气相的相互作用引起的,而不像传统的由强金属-支撑物相互作用(SMSI)引起的自限制封装。最后,我们通过将单晶模型系统与负载pt的TiO2粉末催化剂(P25)直接并排比较,解决了真实催化剂的全部复杂性。我们得出结论,必须仔细选择和控制模型支架的化学计量,以准确地再现氧化还原反应中粉末支架的预期状态。本文章由计算机程序翻译,如有差异,请以英文原文为准。
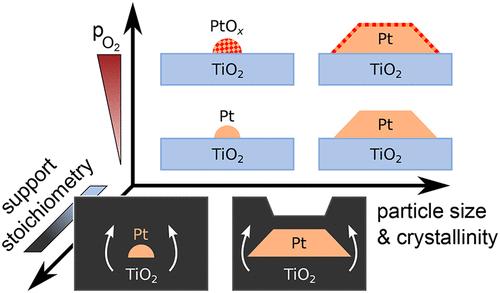
Pt Particles on a Dynamic TiO2 Support in Near-Ambient Conditions–Disentangling Size, Pressure, and Support Effects
Platinum particles on reducible oxides are known to form complex and highly dynamic catalyst systems at elevated pressures and temperatures, often adopting active structures that differ from those found at room temperature and under ultrahigh vacuum (UHV). Here, we study the oxidation and structural evolution of subnanometer Pt clusters and nanoparticles supported on rutile TiO2(110) across an oxygen pressure range from UHV to 0.1 mbar, using near-ambient pressure X-ray photoelectron spectroscopy (NAP-XPS), scanning tunneling microscopy (STM) under UHV and NAP conditions, and low-energy ion scattering (LEIS). Our results reveal distinct differences in oxidation behavior and thermal stability between Pt nanoparticles and clusters, which are further modulated by the support stoichiometry and oxygen pressure. Small Pt clusters become oxidized even at room temperature but are susceptible to accelerated sintering in 0.1 mbar O2 at elevated temperatures. In contrast, well-crystallized Pt nanoparticles on near-stoichiometric TiO2 show weaker oxidation. On a reduced, defective TiO2 support, Pt instead quickly becomes deeply buried by new titania layers, which are formed during support reoxidation. This process appears to result primarily from interactions of the support with the gas phase, unlike the classical, self-limited encapsulation that is induced by the strong metal–support interaction (SMSI). Finally, we address the full complexity of real catalysts in a direct side-by-side comparison of the single-crystalline model system with a Pt-loaded TiO2 powder catalyst (P25). We conclude that the stoichiometry of the model supports must be carefully chosen and controlled to accurately reproduce the expected state of powder supports during redox reactions.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


