利用原位尖端增强拉曼光谱研究Pt(111)上硝基芳烃加氢动力学的机理
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
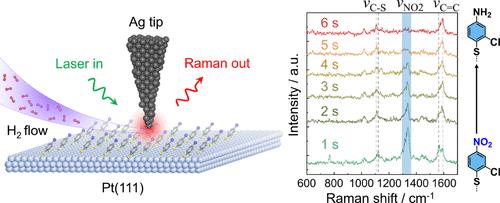
对硝基芳烃在铂上加氢的分子水平动力学机制的了解仍然有限,主要是因为大多数先前的研究依赖于非原位、整体平均测量或孤立考虑的模拟。在这里,我们解决了这一差距,并展示了一种结合原位尖端增强拉曼光谱(TERS)和密度泛函理论(DFT)建模的新方法,以跟踪在一个定义良好的单等离子体结,氯硝基噻吩(CNTP)在原子平面Pt(111)上的加氢。原位TERS捕获了CNTP→氯胺噻吩(CATP)在环境H2暴露下的动态转化,特征时间尺度为~ 6 s。互补DFT模型绘制了反应能量图,揭示了新的机理:CNTP的解吸最初是快速的(势垒0.61 eV),但一旦Pt(111)表面达到一半覆盖范围,解吸速度就会减慢;在半覆盖的Pt(111)表面上的分子弯曲是无障碍的、易受伤害的;第一次加氢到CNTP是很容易的(势垒0.26 eV),而第二次加氢是动力学上要求最高的(势垒0.83 eV),产生的时间尺度为秒,与实验结果相匹配,并确定了速率决定步骤。这些发现促进了对Pt(111)上硝基芳烃加氢的分子水平理解,并证明了原位TERS与第一性原理DFT建模相结合,是纳米尺度上多相催化过程操作机理研究的强大平台。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Mechanistic Insights into Nitroarene Hydrogenation Dynamics on Pt(111) via In Situ Tip-Enhanced Raman Spectroscopy
Mechanistic insights into the molecular-level dynamics of nitroarene hydrogenation on Pt remain limited, largely because most prior studies rely on ex situ, ensemble-averaged measurements, or simulations considered in isolation. Here, we address this gap and demonstrate a novel methodology combining in situ tip-enhanced Raman spectroscopy (TERS) with density functional theory (DFT) modeling to track, at a well-defined single plasmonic junction, the hydrogenation of chloronitrothiophenol (CNTP) on atomically flat Pt(111). In situ TERS captures the dynamic transformation of CNTP → chloroaminothiophenol (CATP) under ambient H2 exposure with a characteristic time scale of ∼6 s. Complementary DFT modeling maps the reaction energetics, revealing novel mechanistic insights: CNTP desorption is rapid initially (barrier 0.61 eV) but slows down once the Pt(111) surface is at about half-coverage; molecular bending on the half-covered Pt(111) surface is barrierless and exergonic; the first hydrogen addition to CNTP is facile (barrier 0.26 eV), while the second hydrogen addition is kinetically most demanding (barrier 0.83 eV), yielding a time scale of seconds that matches experimental results and identifies the rate-determining step. These findings advance molecular-level understanding of nitroarene hydrogenation on Pt(111) and demonstrate in situ TERS integrated with first-principles DFT modeling as a powerful platform for operando mechanistic studies of heterogeneous catalytic processes at the nanoscale.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


