CO和HOCl在空气-水界面反应中自发生成CO2的分子研究
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
化石燃料燃烧和对流层化学中CO的最后一步是通过·OH氧化将其转化为CO2,这是温室气体排放的主要来源,已被广泛研究。然而,对流层中CO-to-CO2转化的机制仍然知之甚少。在此,我们确定了一种新的大气CO2生成途径,该途径由空气-水界面CO和HOCl之间的非均相反应驱动,该反应与·OH无关。利用从头算分子动力学,我们阐明了HOCl的HO部分与CO的C原子配位,形成HOCO中间体,然后是(ii) Cl原子转移到C中心,同时释放HCl和(iii)形成的HCl解离。该反应在约300 K时表现出非常低的自由能垒(ΔGTS = 13.6 kcal mol-1)。更重要的是,ΔGTS随着温度的降低而降低。当温度降至243 K时,反应几乎是自发的(ΔGTS = 1.7 kcal mol-1)。这些发现对进一步理解酸雨的形成和O3耗竭机制具有重要意义。本文章由计算机程序翻译,如有差异,请以英文原文为准。
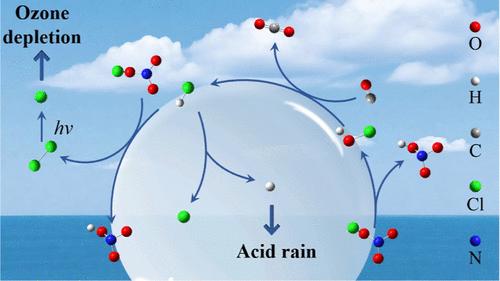
Molecular Insights into the Spontaneous Generation of CO2 in Reaction of CO and HOCl at Air–Water Interfaces
The final step for CO in fossil fuel combustion and tropospheric chemistry is its conversion to CO2 via ·OH oxidation, which represents a major source of greenhouse gas emissions and has been extensively studied. However, the mechanisms for CO-to-CO2 transformation beyond ·OH-mediated pathways in the troposphere remain poorly understood. Herein, we identify a novel atmospheric CO2 generation pathway driven by a heterogeneous reaction between CO and HOCl at air–water interfaces, which operates independently of ·OH. Using ab initio molecular dynamics, we elucidate a stepwise mechanism: (i) coordination of the HO moiety of HOCl with the C atom of CO, forming a HOCO intermediate, followed by (ii) Cl atom transfer to the C center accompanied by simultaneous release of HCl and (iii) dissociation of the formed HCl. The reaction exhibits a remarkably low free-energy barrier (ΔGTS = 13.6 kcal mol–1) at approximately 300 K. More importantly, ΔGTS decreases with decreasing temperature. When the temperature decreases to 243 K, the reaction is almost spontaneous (ΔGTS = 1.7 kcal mol–1). These findings have further important implications for understanding acid rain formation and O3 depletion mechanisms.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


