mettl1介导的fox01 m7G甲基化调节代谢功能障碍相关脂肪肝的脂质代谢
IF 11.9
1区 医学
Q1 ENDOCRINOLOGY & METABOLISM
引用次数: 0
摘要
代谢功能障碍相关脂肪性肝病(MASLD)的特点是肝细胞内脂质积累和变性,其发病机制复杂,使药物开发复杂化。在这项研究中,我们发现甲基转移酶样1 (METTL1)在MASLD小鼠和临床样本的肝脏中上调。肝细胞特异性缺失METTL1抑制脂质合成并促进脂质氧化,减轻高脂肪饮食(HFD)诱导的MASLD小鼠的代谢紊乱。相反,过表达METTL1可以促进脂质合成,同时抑制脂质氧化。m7G测序结果表明,METTL1通过甲基化FoxO1的Exon1区域来调节FoxO1 mRNA的稳定性和蛋白表达水平。此外,我们发现FoxO1的过表达抵消了METTL1缺乏对MASLD小鼠代谢紊乱的保护作用。此外,我们发现了一种有效的METTL1小分子抑制剂,特别是甲基溴Homatropine (HtMBm),可以显著改善hfd诱导的MASLD。总的来说,我们的研究表明METTL1在MASLD的进展中起着至关重要的作用,并强调了靶向METTL1调节这种情况下脂肪酸代谢的治疗潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
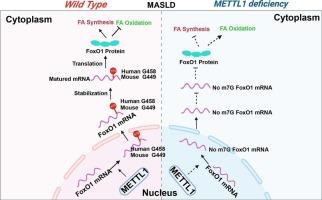
METTL1-mediated m7G methylation of FoxO1 regulates lipid metabolism in metabolic dysfunction-associated fatty liver disease
Metabolic dysfunction-associated fatty liver disease (MASLD) is characterized by the accumulation and degeneration of lipids in hepatocytes, presenting a complex pathogenesis that complicates drug development. In this study, we found that methyltransferase-like 1 (METTL1) is upregulated in the livers of both MASLD mice and clinical samples. Hepatocyte-specific depletion of METTL1 inhibits lipid synthesis and promotes lipid oxidation, alleviating metabolic disorders in high-fat diet (HFD)-induced MASLD mice. Conversely, overexpression of METTL1 enhances lipid synthesis while suppressing lipid oxidation. Mechanistically, METTL1 regulates the stability and protein expression levels of FoxO1 mRNA by methylating the Exon1 region of FoxO1, as demonstrated by m7G sequencing. Additionally, we found that overexpression of FoxO1 counteracts the protective effects of METTL1 deficiency on metabolic disorders in MASLD mice. Moreover, we identified a potent small-molecule inhibitor of METTL1, specifically Homatropine Methylbromide (HtMBm), which significantly ameliorated HFD-induced MASLD. Overall, our study suggests that METTL1 plays a crucial role in the progression of MASLD and highlights the therapeutic potential of targeting METTL1 to modulate fatty acid metabolism in this condition.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Metabolism: clinical and experimental
医学-内分泌学与代谢
CiteScore
18.90
自引率
3.10%
发文量
310
审稿时长
16 days
期刊介绍:
Metabolism upholds research excellence by disseminating high-quality original research, reviews, editorials, and commentaries covering all facets of human metabolism.
Consideration for publication in Metabolism extends to studies in humans, animal, and cellular models, with a particular emphasis on work demonstrating strong translational potential.
The journal addresses a range of topics, including:
- Energy Expenditure and Obesity
- Metabolic Syndrome, Prediabetes, and Diabetes
- Nutrition, Exercise, and the Environment
- Genetics and Genomics, Proteomics, and Metabolomics
- Carbohydrate, Lipid, and Protein Metabolism
- Endocrinology and Hypertension
- Mineral and Bone Metabolism
- Cardiovascular Diseases and Malignancies
- Inflammation in metabolism and immunometabolism
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


