毒伞和豹毒伞的炎症和糖尿病抑制剂:实验和计算结果。
IF 2.2
4区 医学
Q3 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
本研究研究了毛毛伞和黑毛伞中一氧化氮(NO)生成、α-葡萄糖苷酶和蛋白酪氨酸磷酸酶1B (PTP1B)的潜在抑制剂。通过色谱技术和高效液相色谱(HPLC)分离得到16个化合物(1 ~ 16),并通过核磁共振(NMR)和文献比对对其结构进行了鉴定。体外生物活性评价:Griess法对NO抑制作用、对硝基酚法对α-葡萄糖苷酶抑制作用、PTP1B酶促作用。分子对接用于研究蛋白质与配体的相互作用。分离物包括丙氨酸(1)、苏氨酸(2)、2-氨基-4-戊酸(3)、4-羟基-2-吡啶酮(4)、伊博藤酸(5)、5 -羟色胺(6)、尿嘧啶(7)、次黄嘌呤(8)、丁fotenine(9)、β-谷甾醇(10)、豆甾醇(11)、环桉烯醇(12)、spinasterol(13)、daucosterol(14)、豆甾醇-3- o- β- d -葡萄糖苷(15)、白桦林(16)。化合物1和2表现出较弱的NO抑制作用(IC50 = 68.76和52.47 μM)。化合物14对α-葡萄糖苷酶的抑制作用最强(IC50 = 10.56 μM),而化合物10-13和16对α-葡萄糖苷酶的抑制作用中等(IC50 = 19.52 ~ 32.14 μM)。血清素(6)也具有中等活性(IC50 = 78.23 μM)。对于PTP1B的抑制作用,桦木素(16)的抑制作用最强(IC50 = 16.80 μM),其次是化合物10、11和13,其抑制作用较弱(IC50 = 36.28-48.17 μM)。总的来说,所选择的化合物(1,2,10 -14和16)显示出有希望的生物活性,值得进一步对接研究诱导型一氧化氮合酶(iNOS), PTP1B和3AJ7。ADMET(吸收、分布、代谢、排泄和毒性)预测支持有利的药代动力学特性和潜在的药物相似性,强调这些分子是发现抗炎和抗糖尿病药物的先导。本文章由计算机程序翻译,如有差异,请以英文原文为准。
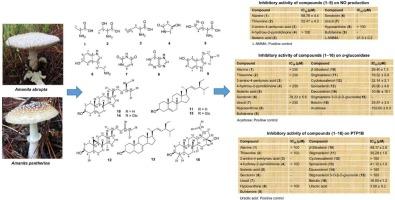
Inflammation and diabetic inhibitors from Amanita abrupta and Amanita pantherina: experimental and computational results
This study investigates potential inhibitors of nitric oxide (NO) production, α-glucosidase, and protein tyrosine phosphatase 1B (PTP1B) from Amanita abrupta and Amanita pantherina. Sixteen compounds (1–16) were isolated by chromatographic techniques and high-performance liquid chromatography (HPLC), and their structures were elucidated by nuclear magnetic resonance (NMR) and literature comparison. Biological activities were evaluated via in vitro assays: the Griess assay for NO inhibition, the p-nitrophenol assay for α-glucosidase, and enzymatic assays for PTP1B. Molecular docking was employed to investigate protein–ligand interactions. The isolates included alanine (1), threonine (2), 2-amino-4-pentynoic acid (3), 4-hydroxy-2-pyrrolidinone (4), ibotenic acid (5), serotonin (6), uracil (7), hypoxanthine (8), bufotenine (9), β-sitosterol (10), stigmasterol (11), cycloeucalenol (12), spinasterol (13), daucosterol (14), stigmasterol-3-O-β-D-glucoside (15), and betulin (16). Compounds 1 and 2 exhibited weak NO inhibition (IC50 = 68.76 and 52.47 μM). Compound 14 displayed the strongest α-glucosidase inhibition (IC50 = 10.56 μM), while compounds 10–13 and 16 showed moderate effects (IC50 = 19.52–32.14 μM). Serotonin (6) also had moderate activity (IC50 = 78.23 μM). For PTP1B inhibition, betulin (16) was most potent (IC50 = 16.80 μM), followed by compounds 10, 11, and 13 with weaker activities (IC50 = 36.28–48.17 μM). Overall, selected compounds (1, 2, 10–14, and 16) exhibited promising bioactivities, warranting further docking studies against inducible nitric oxide synthase (iNOS), PTP1B, and 3AJ7. ADMET (absorption, distribution, metabolism, excretion, and toxicity) predictions supported favorable pharmacokinetic properties and potential drug-likeness, highlighting these molecules as leads for anti-inflammatory and anti-diabetic drug discovery.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
5.70
自引率
3.70%
发文量
463
审稿时长
27 days
期刊介绍:
Bioorganic & Medicinal Chemistry Letters presents preliminary experimental or theoretical research results of outstanding significance and timeliness on all aspects of science at the interface of chemistry and biology and on major advances in drug design and development. The journal publishes articles in the form of communications reporting experimental or theoretical results of special interest, and strives to provide maximum dissemination to a large, international audience.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


