Mn2+掺杂SrTiO3纳米晶体的胶体合成:b位取代和自旋弛豫动力学
IF 3.2
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
我们报道了一种独特的纳米晶体(NC)体系的胶体水热合成,其中Mn2+离子被引入SrTiO3,并取代了八面体Ti4+位点,而不是固态合成中报道的a位点。这一观察结果得到了电子顺磁谱的支持,其中Mn2+信号具有超精细的分裂值,与大块SrTiO3中B位的Mn2+更一致。我们也没有通过任何光谱方法在Mn:SrTiO3 nc中检测到任何高价的Mn3+或Mn4+。采用连续波EPR功率饱和研究方法,在100 K下定性地研究了Mn2+掺杂剂在SrTiO3纳米材料中引入Ti3+缺陷前后的自旋弛豫动力学。我们观察到室温下Mn2+ EPR线宽度的加宽与Ti3+和Mn2+之间的交叉弛豫一致。这一结果与我们之前在光掺杂Cr3+:SrTiO3 NCs后加速Cr3+自旋弛豫的演示相似,但效率较低。这种较弱的交叉弛豫是由于land本文章由计算机程序翻译,如有差异,请以英文原文为准。
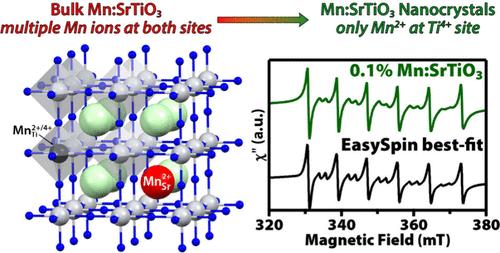
Colloidal Synthesis of Mn2+-Doped SrTiO3 Nanocrystals: B-Site Substitution and Spin-Relaxation Dynamics
We report the colloidal hydrothermal synthesis of a unique nanocrystal (NC) system where Mn2+ ions are introduced into SrTiO3 and appear to substitute at the octahedral Ti4+ site instead of the A site that has been reported from solid-state syntheses. This observation is supported by electron paramagnetic spectroscopy, where the Mn2+ signal has a hyperfine splitting value that is more consistent with Mn2+ at the B site in bulk SrTiO3. We also do not detect any higher-valent Mn3+ or Mn4+ by any spectroscopic method in the Mn:SrTiO3 NCs. The spin-relaxation dynamics of the Mn2+ dopants before and after introducing Ti3+ defects into the SrTiO3 NCs through photodoping is also studied qualitatively using continuous-wave EPR power saturation studies at 100 K. We observe broadening of the Mn2+ EPR line widths consistent with cross-relaxation between Ti3+ and Mn2+ up to room temperature. This result is similar to but less efficient than our previous demonstration of accelerated spin relaxation of Cr3+ after photodoping Cr3+:SrTiO3 NCs. This weaker cross-relaxation results from the larger difference in Landé g factors that results in a larger energy mismatch between the Mn2+ and Ti3+ EPR transitions in an applied magnetic field.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

The Journal of Physical Chemistry C
化学-材料科学:综合
CiteScore
6.50
自引率
8.10%
发文量
2047
审稿时长
1.8 months
期刊介绍:
The Journal of Physical Chemistry A/B/C is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, and chemical physicists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


