烷基碘化物的无金属羰基化和无co羰基化
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
本文报道了一种在光化学条件下羰基化碘化烷基的新方法。该过程依赖于一种简单的甲酸芳基试剂,由现成的化学物质一步合成,能够在温和的碱存在下释放CO和酚酸盐。这种酚酸盐的光活性被利用来通过单电子转移激活烷基碘化物,并产生烷基自由基,烷基自由基增加CO,最终提供羧酸和酰胺的通道。这种新的,用户友好的程序克服了羰基化化学中对CO气体和/或金属催化剂的典型需求,同时具有高效率和通用性,可容纳各种官能团。本文章由计算机程序翻译,如有差异,请以英文原文为准。
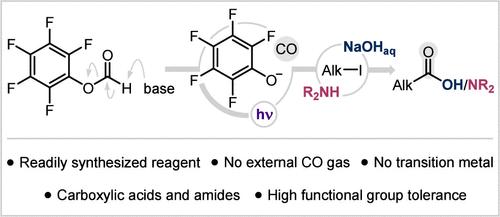
Metal- and CO-Free Carbonylation of Alkyl Iodides
We report a new method for the carbonylation of alkyl iodides under photochemical conditions. The process relies on a simple aryl formate reagent, synthesized in one step from readily available chemicals and capable of releasing CO along with a phenolate species in the presence of a mild base. The photoactivity of this phenolate is harnessed to activate alkyl iodides via single-electron transfer and generate alkyl radicals that add to CO and ultimately offer access to carboxylic acids and amides. This new, user-friendly procedure overcomes the typical need for CO gas and/or metal catalysts in carbonylation chemistry while being highly efficient and versatile to accommodate a variety of functional groups.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


