白屈酸激活FoxO3a/PINK1/Parkin信号通路,抑制慢性间歇性缺氧诱导的心脏氧化应激、炎症反应和NLRP3炎性小体活化。
IF 5.4
2区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
阻塞性睡眠呼吸暂停综合征(OSAS)引起的心脏损伤与慢性间歇性缺氧(CIH)密切相关,但其分子机制和潜在的干预策略有待进一步探索。白屈酸(AA)是一种从橡苔中提取的化合物,因其抗炎和抗氧化特性而备受关注。然而,在CIH背景下,其对心脏保护作用的保护作用尚未被系统地研究。通过建立CIH小鼠模型和H9C2细胞IH损伤模型,结合组织病理学分析、线粒体功能评估、qPCR、免疫荧光、Western blot等方法,评价AA对CIH诱导心肌损伤的保护作用及分子机制。实验组包括不同剂量的对照组、CIH组和AA干预组。AA处理可显著减轻cih诱导的心脏损伤,抑制ROS积累、线粒体功能障碍和氧化应激,并通过促进FoxO3a核易位激活Pink1/Parkin途径介导的线粒体自噬。同时,AA抑制NLRP3炎性体的激活和炎症反应。AA的保护作用被自噬抑制或ROS增强逆转,提示有丝自噬与炎症调节之间存在相互作用机制。本研究首次证明,AA通过FoxO3a-PINK1/Parkin途径增强线粒体自噬,同时协同抑制氧化应激和NLRP3炎性体激活,从而减轻cih相关的心肌损伤。这些发现为osas相关心脏并发症提供了新的治疗靶点,并为AA的临床转化奠定了理论基础,但需要进一步的临床验证。本文章由计算机程序翻译,如有差异,请以英文原文为准。
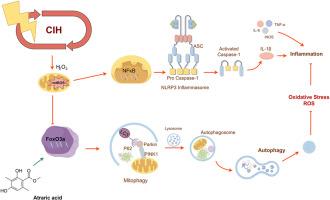
Atraric acid activates the FoxO3a/PINK1/Parkin signaling pathway to suppress chronic intermittent hypoxia-induced cardiac oxidative stress, inflammatory responses, and NLRP3 inflammasome activation
Obstructive sleep apnea syndrome (OSAS)-induced cardiac injury is closely associated with chronic intermittent hypoxia (CIH), but its molecular mechanisms and potential intervention strategies require further exploration. Atraric acid (AA), a compound extracted from oakmoss, has garnered attention due to its anti-inflammatory and antioxidant properties. However, its protective effects on the cardioprotective effects in the context of CIH have not been systematically investigated. Through the establishment of CIH mouse models and H9C2 cell IH injury models, combined with histopathological analysis, mitochondrial function assessment, qPCR, immunofluorescence, and Western blot, the protective effects and molecular mechanisms of AA against CIH-induced myocardial injury were evaluated. Experimental groups included a control group, CIH group, and AA intervention groups with varying doses. AA treatment significantly alleviated CIH-induced cardiac damage, suppressed ROS accumulation, mitochondrial dysfunction, and oxidative stress, and activated Pink1/Parkin pathway-mediated mitophagy by promoting FoxO3a nuclear translocation. Meanwhile, AA inhibited NLRP3 inflammasome activation and inflammatory responses. The protective effects of AA were reversed by autophagy inhibition or ROS enhancement, suggesting an interaction mechanism between mitophagy and inflammation regulation. This study is the first to demonstrate that AA mitigates CIH-related myocardial injury by enhancing mitophagy via the FoxO3a-PINK1/Parkin pathway while synergistically suppressing oxidative stress and NLRP3 inflammasome activation. These findings provide novel therapeutic targets for OSAS-associated cardiac complications and establish a theoretical foundation for the clinical translation of AA, though further clinical validation is warranted.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.70
自引率
3.90%
发文量
410
审稿时长
36 days
期刊介绍:
Chemico-Biological Interactions publishes research reports and review articles that examine the molecular, cellular, and/or biochemical basis of toxicologically relevant outcomes. Special emphasis is placed on toxicological mechanisms associated with interactions between chemicals and biological systems. Outcomes may include all traditional endpoints caused by synthetic or naturally occurring chemicals, both in vivo and in vitro. Endpoints of interest include, but are not limited to carcinogenesis, mutagenesis, respiratory toxicology, neurotoxicology, reproductive and developmental toxicology, and immunotoxicology.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


