熔铝酸盐的切割和缝合策略:两种不同的烯烃的磺酰甲基化接力获得内酰胺-色素杂化烷基-烷基砜
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
在过去的几十年里,不同烯烃的自由基接力反应已经成为构建功能分子的关键策略。然而,开发可以同时连接两个不同烯烃的官能团仍然是一个重大挑战。在此,我们报道了一个有效的融铝石诱导的n -烯丙基溴乙酰胺与邻羟基胺酮的磺酰甲基化继电反应,使用融铝石作为“SO2”和“C1”源,得到内酰胺-色素杂化烷基-烷基砜。值得注意的是,这种转化采用了一种新颖的“切割-缝合”策略,将熔铝石“切割”成硫和碳碎片,随后与两种不同的烯烃进行选择性的“缝合”,以获得目标产品。这种磺基甲基化接力反应具有高效率、广泛的底物耐受性和良好的可扩展性,能够将产物转化为有价值的杂环骨架。机理研究表明,该反应是通过自由基途径进行的,熔铝石具有三重作用:作为超级电子供体引发反应,同时作为“SO2”和“C1”源。本文章由计算机程序翻译,如有差异,请以英文原文为准。
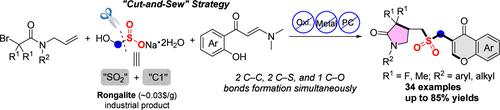
A Cut-and-Sew Strategy with Rongalite: Sulfonylmethylation Relay of Two Distinct Alkenes to Access Lactam–Chromone Hybrid Alkyl–Alkyl Sulfones
Over the past few decades, radical relay reactions of distinct alkenes have emerged as a pivotal strategy for constructing functional molecules. However, developing functional groups that can link two distinct alkenes simultaneously remains a significant challenge. Herein, we report an efficient rongalite-induced sulfonylmethylation relay reaction of N-allylbromoacetamides with o-hydroxyaryl enaminones using rongalite as the “SO2” and “C1” sources to access lactam–chromone hybrid alkyl–alkyl sulfones. Notably, this transformation employs a novel “cut-and-sew” strategy, where rongalite is “cut” into sulfur and carbon fragments, which subsequently undergo selective “sew” with two distinct alkenes to access the target product. This sulfonylmethylation relay reaction demonstrates high efficiency, broad substrate tolerance, and excellent scalability and enables the conversion of products into valuable heterocyclic skeletons. Mechanistic studies revealed that this reaction proceeded through a radical pathway, with rongalite serving triple roles: as a super electron donor to initiate the reaction, as both “SO2” and “C1” sources.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


