喹啉序贯脱芳法制备全苯胺内酯B
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
我们报道了第一个含环丁烷的石蒜生物碱酯内酯b的全合成。我们的方法利用顺序喹啉去芳化策略,使这种容易获得的杂芳香前体转化为三环n -酰基二氢吡啶酮中间体。广泛的研究旨在锻造签名环丁烷揭示了β-丙烯酸硅酯是关键的[2 + 2]光环加成反应的成功至关重要。进一步的官能团转化和最终的一锅甲基化/内酯化序列在20步内得到注释内酯B。本文章由计算机程序翻译,如有差异,请以英文原文为准。
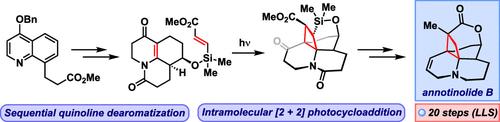
Total Synthesis of Annotinolide B via Sequential Quinoline Dearomatization
We report the first total synthesis of the complex cyclobutane-containing Lycopodium alkaloid annotinolide B. Our approach leverages a sequential quinoline dearomatization strategy that enables the transformation of this readily accessible heteroaromatic precursor to a tricyclic N-acyl dihydropyridone intermediate. Extensive studies aimed at forging the signature cyclobutane revealed a β-silyl acrylate to be essential for the success of a key [2 + 2] photocycloaddition reaction. Further functional group transformations and a final one-pot methylation/lactonization sequence furnish annotinolide B in 20 steps.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


