暂时钼的η - 6配位使苯的1,2-脱芳和一般加氢功能化成为可能
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
尽管钼在有机合成中具有可持续发展的特点和广泛的用途,但其在高价值芳烃脱芳功能化中的应用仍未得到充分开发。在此,我们报道了通过瞬态钼的η - 6配位对非活化芳烃进行一般和有效的1,2-加氢功能化。该方法在温和的条件下操作,具有广泛的底物范围和高区域选择性,从而方便地获得有价值的功能化1,3-环己二烯。钼活化允许在复杂的多芳系统中精确的环选择性脱芳,并促进药物分子的后期修饰。通过(±)-感染石蜡酮的全合成和(±)-菊苣酮和(±)-石蜡烷的正式合成,进一步证明了其合成的实用性。值得注意的是,与传统的铬类似物相比,钼基体系表现出独特的反应活性和优越的选择性,突出了其独特的选择性脱芳功能化能力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
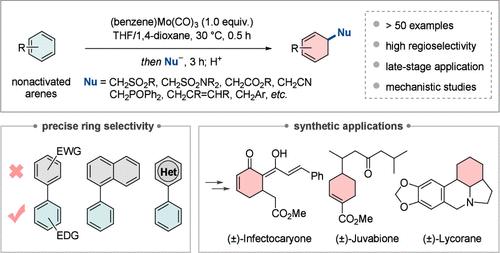
Temporary Molybdenum η6-Coordination Enables Selective and General Dearomative 1,2-Hydrofunctionalization of Benzenes
Despite molybdenum’s sustainable profile and broad utility in organic synthesis, its use in high-value arene dearomative functionalizations remains markedly underexplored. Herein, we report a general and efficient dearomative 1,2-hydrofunctionalization of nonactivated arenes via transient molybdenum η6-coordination. This method operates under mild conditions and exhibits broad substrate scope and high regioselectivity, thus providing convenient access to valuable functionalized 1,3-cyclohexadienes. The molybdenum activation allows precise ring-selective dearomatization within complex polyaromatic systems and facilitates late-stage modification of drug molecules. Its synthetic utility is further demonstrated through the total synthesis of (±)-infectocaryone and formal syntheses of (±)-juvabione and (±)-lycorane. Notably, the molybdenum-based system demonstrates unique reactivity and superior selectivity compared to conventional chromium analogues, highlighting its distinct capability for selective dearomative functionalization.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


