肿瘤浸润细菌破坏癌上皮细胞的相互作用并诱导细胞周期阻滞
IF 44.5
1区 医学
Q1 CELL BIOLOGY
引用次数: 0
摘要
肿瘤浸润细菌越来越被认为是癌症进展和治疗耐药性的调节剂。我们描述了细胞外肿瘤内细菌,包括梭杆菌,调节癌症上皮细胞行为的机制。空间成像和单细胞空间转录组学显示,这些细菌主要定位于结直肠癌和口腔癌的肿瘤微生境内的细胞外,其特征是细胞密度、转录活性和增殖降低。体外,核梭杆菌破坏上皮接触,诱导G0-G1阻滞和转录静止。这种状态赋予5-氟尿嘧啶耐药性并重塑肿瘤微环境。研究结果通过活细胞成像、空间分析、小鼠模型和52例结直肠癌患者队列验证。转录组学揭示了细菌富集区细胞周期、转录和抗原呈递基因的下调,与静止、免疫逃避表型一致。在一个独立的直肠癌队列中,高梭杆菌负荷与治疗反应降低相关。这些结果将细胞外细菌与癌细胞静止和化疗耐药联系起来,突出了微生物-肿瘤相互作用作为治疗靶点。本文章由计算机程序翻译,如有差异,请以英文原文为准。
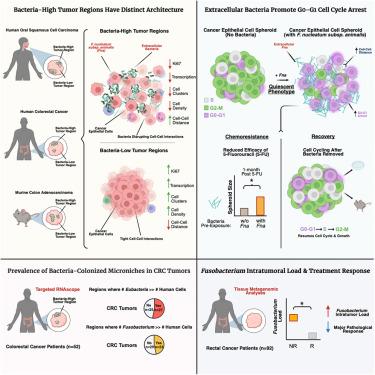
Tumor-infiltrating bacteria disrupt cancer epithelial cell interactions and induce cell-cycle arrest
Tumor-infiltrating bacteria are increasingly recognized as modulators of cancer progression and therapy resistance. We describe a mechanism by which extracellular intratumoral bacteria, including Fusobacterium, modulate cancer epithelial cell behavior. Spatial imaging and single-cell spatial transcriptomics show that these bacteria predominantly localize extracellularly within tumor microniches of colorectal and oral cancers, characterized by reduced cell density, transcriptional activity, and proliferation. In vitro, Fusobacterium nucleatum disrupts epithelial contacts, inducing G0-G1 arrest and transcriptional quiescence. This state confers 5-fluorouracil resistance and remodels the tumor microenvironment. Findings were validated by live-cell imaging, spatial profiling, mouse models, and a 52-patient colorectal cancer cohort. Transcriptomics reveals downregulation of cell cycle, transcription, and antigen presentation genes in bacteria-enriched regions, consistent with a quiescent, immune-evasive phenotype. In an independent rectal cancer cohort, high Fusobacterium burden correlates with reduced therapy response. These results link extracellular bacteria to cancer cell quiescence and chemoresistance, highlighting microbial-tumor interactions as therapeutic targets.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cancer Cell
医学-肿瘤学
CiteScore
55.20
自引率
1.20%
发文量
179
审稿时长
4-8 weeks
期刊介绍:
Cancer Cell is a journal that focuses on promoting major advances in cancer research and oncology. The primary criteria for considering manuscripts are as follows:
Major advances: Manuscripts should provide significant advancements in answering important questions related to naturally occurring cancers.
Translational research: The journal welcomes translational research, which involves the application of basic scientific findings to human health and clinical practice.
Clinical investigations: Cancer Cell is interested in publishing clinical investigations that contribute to establishing new paradigms in the treatment, diagnosis, or prevention of cancers.
Insights into cancer biology: The journal values clinical investigations that provide important insights into cancer biology beyond what has been revealed by preclinical studies.
Mechanism-based proof-of-principle studies: Cancer Cell encourages the publication of mechanism-based proof-of-principle clinical studies, which demonstrate the feasibility of a specific therapeutic approach or diagnostic test.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


