水溶液锌离子电池六方硫化镉阴极:硫化锌形成及锌/镉电池稳定性研究
IF 3.2
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
本文报道了二硫系硫化镉(CdS)的易共沉淀法合成,该材料已被用作锌离子电池的高效阴极。采用碱性ph共沉淀法合成了纯相六方CdS。利用相关的最新技术研究了单电极锌/镉电池的结构性能、电子性能以及形态和电化学分析。采用循环伏安法对阴极CdS和阳极Zn进行波段对准,确定了阴极CdS与Zn的相容性。由于Zn2+离子的插层作用,ZnS在阴极放电形成过程中,Zn/CdS体系获得了1.45的高工作电位。采用Born-Haber方法和解释Zn-S键形成的广义kapusstinskii方程研究了键的形成和内聚力。对具有~ 159 mAh/g高容量的Zn/CdS体系进行了恒流充放电(GCD)循环性能分析。本文章由计算机程序翻译,如有差异,请以英文原文为准。
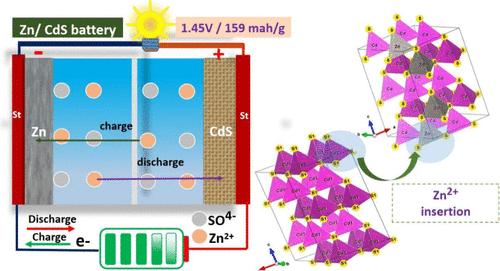
Hexagonal Cadmium Sulfide Cathode for Aqueous Zinc-Ion Battery: Study of Zinc-Sulfide Formation and Zn/CdS Battery Stability
We report facile coprecipitation synthesis of binary chalcogenide cadmium sulfide (CdS), which has been used as an efficient cathode for aqueous zinc-ion batteries. Pure phase hexagonal CdS was synthesized using an alkaline pH-based coprecipitation method. Structural properties, electronic properties and morphological and electrochemical analysis of the single electrodes’ & Zn/CdS cell performance were studied using relevant state-of-the-art techniques. Determination of the compatibility of the cathode CdS with the Zn anode was carried out by band alignment of the cathode CdS and anode Zn, which was studied by cyclic voltammetric analysis. The high operating potential of 1.45 was obtained for the Zn/CdS system, during the discharge formation of ZnS, which occurs at the cathode because of the Zn2+ cation intercalation. The bond formation and cohesive forces were studied by the Born–Haber approach and the generalized Kapustinskii equation explaining the formation of Zn–S bonds. The galvanostatic charge–discharge (GCD) cyclic performance analysis was carried out for the Zn/CdS system with a high capacity of ∼159 mAh/g.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

The Journal of Physical Chemistry C
化学-材料科学:综合
CiteScore
6.50
自引率
8.10%
发文量
2047
审稿时长
1.8 months
期刊介绍:
The Journal of Physical Chemistry A/B/C is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, and chemical physicists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


