揭示纳米结构锐钛矿TiO2存储Li+的界面化学和动力学
IF 3.2
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
二氧化钛(TiO2)虽然是一种经济且稳定的锂离子电池负极材料,但其锂离子插入动力学有限。为了克服这一点,我们通过合成三种不同的锐钛矿TiO2单晶形貌(立方、截形和八面体),并控制暴露在{001}、{100}和{101}面来全面研究facet依赖的电化学动力学。电化学表征、速率测试、扩散系数分析、阻抗谱和循环后结构探针表明,与以{101}面为主的八面体颗粒相比,富含高能{001}和{100}面的形貌具有增强的可逆容量保留和更高的有效Li+扩散系数。我们证明这些改进来自于表面稳定和更少的寄生反应。除了二氧化钛,这项工作还建立了一种可转移的、以材料为中心的方法,结合多模态电化学和结构询问的面控合成,用于分离和理解任何新兴电极材料中的结构-传输关系。这些机制上的见解重新定义了面工程作为设计高速率、长寿命插入电极的主动策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
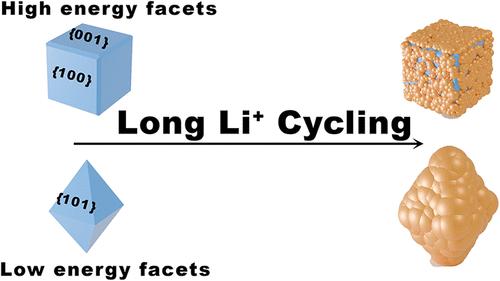
Unveiling the Facet-Dependent Interfacial Chemistry and Kinetics in Nanostructured Anatase TiO2 for Li+ Storage
Titanium dioxide (TiO2), while being an affordable and stable anode material for Li-ion batteries, suffers from limited Li+ insertion kinetics. To overcome this, we comprehensively investigate the facet-dependent electrochemical dynamics by synthesizing three distinct single-crystal anatase TiO2 morphologies (cubic, truncated, and octahedral) with controlled exposure of {001}, {100}, and {101} facets. Combined electrochemical characterizations, rate testing, diffusion coefficient analysis, impedance spectroscopy, and postcycling structural probes reveal that morphologies enriched in high-energy {001} and {100} facets, cubic and truncated, show enhanced reversible capacity retention and higher effective Li+ diffusion coefficients compared with octahedral particles dominated by {101} facets. We show that these improvements arise from surface stabilization and less parasitic reactions. Beyond titania, this work establishes a transferable, materials-centric approach, facet-controlled synthesis coupled with multimodal electrochemical and structural interrogation, for isolating and understanding structure–transport relationships in any emerging electrode materials. These mechanistic insights reframe facet engineering as an active strategy for designing high-rate, long-life intercalation electrodes.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

The Journal of Physical Chemistry C
化学-材料科学:综合
CiteScore
6.50
自引率
8.10%
发文量
2047
审稿时长
1.8 months
期刊介绍:
The Journal of Physical Chemistry A/B/C is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, and chemical physicists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


