新型氟化二氢吡喃萘醌化合物的改进合成及其抗炎活性。
IF 2.2
4区 医学
Q3 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
采用微波四组份反应,以lawson、氟化芳醛、4,4,4-三氟乙酸乙酯和乙酸铵为原料,合成了新的氟化二氢吡萘醌类化合物。通过光谱方法对产物进行了全面表征,并对脂多糖刺激的RAW264.7巨噬细胞进行了抗炎活性评估。所有化合物均具有显著的抑制NO生成作用,IC50值在1.67 ± 0.02 ~ 28.81 ± 2.44 μM之间,无明显毒性。有趣的是,化合物8n对脂多糖(LPS)刺激的RAW264.7巨噬细胞的NO抑制活性最强,IC50值为1.67 ± 0.02 μM。此外,化合物8c、h、i、l显著降低lps刺激RAW264.7巨噬细胞中促炎因子IL-1β和IL-6的水平。这些结果表明这些化合物具有开发新型抗炎药的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
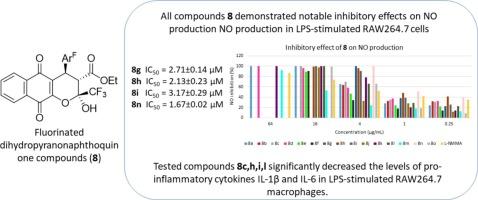
Improved synthesis and anti-inflammatory activity of new fluorinated dihydropyranonaphthoquinone compounds
This study presents the synthesis of new fluorinated dihydropyranonapthoquinone compounds by means of microwave-prompted four-component reactions using lawson, fluorinated aromatic aldehydes, ethyl 4,4,4-trifluoroacetoacetate and ammonium acetate. The products were thoroughly characterized by spectroscopic methods and assessed for their anti-inflammatory activity in lipopolysaccharide-stimulated RAW264.7 macrophage cells. All synthesized compounds demonstrated notable inhibitory effects on NO production with IC50 ranging from 1.67 ± 0.02 to 28.81 ± 2.44 μM without causing significant toxicity. Interestingly, compound 8n showed the most potent NO inhibitory activity in lipopolysaccharide (LPS)-stimulated RAW264.7 macrophage cells with IC50 value of 1.67 ± 0.02 μM. Moreover, compounds 8c,h,i,l significantly decreased the levels of pro-inflammatory cytokines IL-1β and IL-6 in LPS-stimulated RAW264.7 macrophages. These results indicate the potential of these compounds as promising candidates for the development of new anti-inflammatory agents.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
5.70
自引率
3.70%
发文量
463
审稿时长
27 days
期刊介绍:
Bioorganic & Medicinal Chemistry Letters presents preliminary experimental or theoretical research results of outstanding significance and timeliness on all aspects of science at the interface of chemistry and biology and on major advances in drug design and development. The journal publishes articles in the form of communications reporting experimental or theoretical results of special interest, and strives to provide maximum dissemination to a large, international audience.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


