氟稳定超疏水铜(δ+)提高CO2还原C2+产物法拉第效率
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
氧化物衍生(OD) Cu催化剂因其生产C2+产物的有效性而得到认可,但由于失去了正氧化态,通常会还原为金属态,从而降低了选择性。在这里,我们报告了一种使用氢氟酸(HF)将氟(F)掺入Cu2O纳米球的新策略。卤素酸传统上被用于蚀刻金属和制造约束效应的空腔,而这项工作更进一步,将F离子插入晶格中。在所有卤素中,F具有最好的晶格稳定性,因此被选择用于催化剂测试。F的掺入稳定了Cu (δ+)的氧化态,在电流密度为250 mA cm-2时,C2+产物的法拉第效率(FE)达到了91.9±2.03%,主要是乙烯(67%)。高场1D和2D 19F魔角旋转(MAS)固体核磁共振(ssNMR)谱提供了明确的证据,证明了F在氧空位上取代和HF表面层的形成。水接触角(WCA)测量结果显示,3 M hf处理的Cu2O具有超疏水性(WCA = 161°),有效抑制了析氢(HER)。原位拉曼光谱证实了f -催化剂中Cu2O相的长期稳定性,而带有同位素标记的原位ATR-FTIR光谱则阐明了乙烯生成的机理途径。此外,密度泛函理论(DFT)计算提供了乙烯形成的机理见解,而Bader电荷分析揭示了F在Cu2O中结合的电子作用,从而为其增强的选择性提供了见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
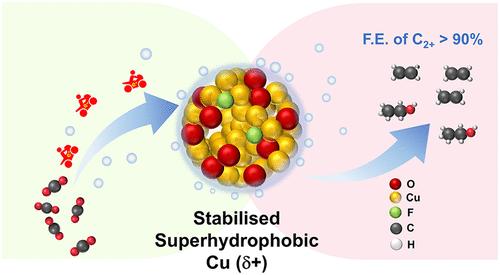
Enhancing C2+ Product Faradaic Efficiency in CO2 Reduction Using Fluorine-Stabilized Superhydrophobic Copper (δ+)
Oxide-derived (OD) Cu catalysts are recognized for their effectiveness in producing C2+ products, but often revert to their metallic state, reducing selectivity due to the loss of their positive oxidation state. Here, we report a novel strategy to incorporate fluorine (F) into Cu2O nanospheres using hydrofluoric acid (HF). While halogen acids have traditionally been employed to etch metals and create cavities for confinement effects, this work goes a step further by inserting F ions into the lattice. Among all halogens, F provided the best lattice stability and was thus selected for catalyst testing. F incorporation was found to stabilize the Cu (δ+) oxidation state, achieving an impressive Faradaic efficiency (FE) of 91.9 ± 2.03% for C2+ products, predominantly ethylene (67%), at a current density of 250 mA cm–2. High-field 1D and 2D 19F magic-angle spinning (MAS) solid-state nuclear magnetic resonance (ssNMR) spectroscopy provided definitive evidence of F substitution at oxygen vacancies and the formation of a surface layer of HF. Water contact angle (WCA) measurements revealed enhanced hydrophobicity, with the 3 M HF-treated Cu2O exhibiting superhydrophobicity (WCA = 161°), which effectively suppressed hydrogen evolution (HER). In situ Raman spectroscopy confirmed prolonged stability of the Cu2O phase in the F-incorporated catalyst, while in situ ATR-FTIR spectroscopy with isotopic labeling elucidated the mechanistic pathway for ethylene production. Additionally, density functional theory (DFT) calculations offered mechanistic insights into ethylene formation, while Bader charge analysis revealed the electronic role of F incorporation in Cu2O, thereby providing insight into its enhanced selectivity.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


