钯/降冰片烯协同催化“从头”构建平面手性二茂铁
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
过渡金属催化的不对称碳氢功能化已成为构建平面手性二茂铁(PCFs)的一种有效策略。然而,目前的方法通常需要预先安装一个定向组(DG),并且通常仅限于单功能化。本文报道了一种独特的(C1,C4)桥头堡修饰的手性降冰片烯作为助催化剂和唯一的手性源,通过Pd/NBE*协同催化“从头开始”构建PCFs。这种方法可以使简单的二茂碘铁在一个步骤中形成两个新的化学键。以高达99%的ee快速合成了一系列平面手性异喹诺酮熔合二茂铁。机理研究表明,邻位碳氢活化步骤是对映体的决定步骤,而还原消除不仅是对映体的决定步骤,而且在进一步提高对映体选择性方面起着关键作用。合成用途是突出的多样化的转化产品和开发一个强大的NAD(P)H辅酶的烯烃仿生不对称氢化。本文章由计算机程序翻译,如有差异,请以英文原文为准。
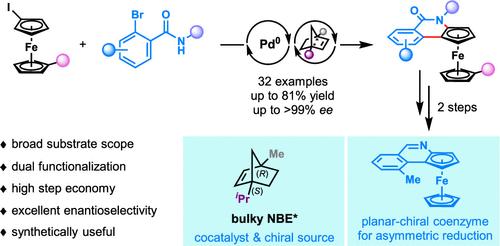
“De Novo” Construction of Planar-Chiral Ferrocenes via Palladium/Chiral Norbornene Cooperative Catalysis
Transition-metal-catalyzed asymmetric C–H functionalization has emerged as a powerful strategy for constructing planar-chiral ferrocenes (PCFs). However, current methods typically require preinstallation of a directing group (DG) and are usually limited to monofunctionalization. Herein, we report a “de novo” construction of PCFs via Pd/NBE* cooperative catalysis, employing a unique bulky (C1,C4)-bridgehead-modified chiral norbornene as the cocatalyst as well as the only chiral source. This approach enables the formation of two new chemical bonds in a single step from simple iodoferrocenes. A series of planar-chiral isoquinolone-fused ferrocenes are quickly obtained with up to >99% ee. Mechanistic studies reveal that the ortho-C–H activation step serves as the enantio-determining step, while the reductive elimination is not only the rate-determining step but also plays a pivotal role in further enhancing the enantioselectivity. The synthetic utility is highlighted by diverse transformations of products and the development of a powerful NAD(P)H coenzyme for biomimetic asymmetric hydrogenation of olefins.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


