混合离子-共价键实现持久的酸性水氧化
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
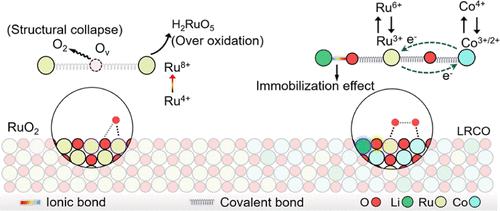
在酸性条件下,析氧反应(OER)催化剂的不稳定性阻碍了质子交换膜(PEM)电解的广泛应用,这源于耦合降解机制:晶格氧过度氧化(由高金属-氧(M-O)共价驱动)和酸诱导的金属浸出/崩溃。在这里,我们介绍了一种电负性引导的Li和Ru掺杂尖晶石Co3O4 (LRCO)催化剂,具有Li- o(离子)和Ru/ Co-O(共价键)混合键。该设计同时通过强Li-O锚定稳定晶格氧,并通过Ru-O-Co电子共享调节金属价态,防止过度氧化。Operando研究揭示了一种电位依赖机制,其中Ru在低电位下向Co提供电子,形成活性Ru物质,电子再分配防止Ru在高电位下过度氧化。至关重要的是,Li-O键在整个过程中有效地抑制了氧空位的形成,并且即使在高电位下,Ru-O配位也保持稳定。LRCO在10 mA cm-2下实现了创纪录的低过电位141 mV,稳定性>;3,300 h。集成PEM电解槽在1 A cm-2下运行720小时,实现了下一个十年工业目标的关键里程碑。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Mixed Ionic–Covalent Bonds Achieve Durable Acidic Water Oxidation
The widespread deployment of proton exchange membrane (PEM) water electrolysis is hindered by the instability of oxygen evolution reaction (OER) catalysts under acidic conditions, stemming from coupled degradation mechanisms: lattice oxygen overoxidation (driven by high metal–oxygen (M–O) covalency) and acid-induced metal leaching/collapse. Here, we introduce an electronegativity-guided Li- and Ru-doped spinel Co3O4 (LRCO) catalyst featuring mixed Li–O (ionic) and Ru/Co–O (covalent) bonding. This design simultaneously stabilizes lattice oxygen via strong Li–O anchoring and modulates metal valence states through Ru–O–Co electron sharing, preventing overoxidation. Operando studies reveal a potential-dependent mechanism where Ru donates electrons to Co at low potentials, forming active Ru species, and electron redistribution prevents Ru overoxidation at high potentials. Crucially, Li–O bonds effectively suppress oxygen vacancy formation throughout, and Ru–O coordination remains stable, even at high potentials. LRCO achieves a record-low overpotential of 141 mV at 10 mA cm–2 with >3,300 h stability. Integrated PEM electrolyzers operated >720 h at 1 A cm–2, achieving a key milestone toward next-decade industry targets.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


