铜分子配合物的合成、结构研究及电催化水氧化性能:配体n -烷基化和配位数的影响
IF 4.7
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
在本研究中,我们报道了三种以2-吡啶基甲基取代1,4,7-三氮杂环壬烷为配体的新型铜配合物的合成、结构研究和电催化水氧化性能。结构表征揭示了配合物[Cu(Py2tcan)](ClO4)2 (1, Py2tcan = 1,4-二(2-吡啶基)-1,4,7-三氮杂环壬烷)和配合物[Cu(MePy2tcan)](ClO4)2 (2, MePy2tcan = 1-甲基-4,7-二(2-吡啶基)-1,4,7-三氮杂环壬烷)的五配位结构,以及配合物[Cu(Py3tcan)](ClO4)2 (3, Py3tcan = 1,4,7-三氮杂环壬烷)的六配位结构。系统的电化学分析表明,1和2对水的氧化具有较高的催化效率。机理研究表明,1中的仲胺配位通过增强σ给体效应显著降低了催化过电位,从而促进Cu中心氧化,加快了催化循环。而2的配位环境中含有两个吡啶基团和三个叔胺基团,由于其N-CH3结构的σ给体性质较差,其催化过电位高于1。此外,3表现出较低的催化活性,强调了不饱和配位几何结构对有效水氧化的必要性。与类似的Ni配合物[Ni(Py3tcan)]2+(4)和Fe配合物[Fe(Py3tcan)]2+相比,Cu中心表现出更高的起始过电位和更低的催化活性,这表明即使用相同的Py3tacn配体稳定,Cu中心对水氧化的内在催化活性也不如Ni和Fe中心。通过合理调节配体结构和配位数,可以提高铜配合物的催化性能。本文章由计算机程序翻译,如有差异,请以英文原文为准。
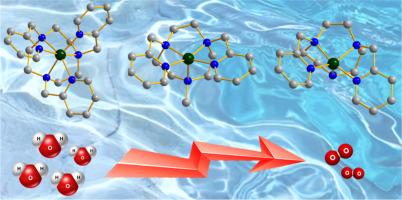
Synthesis, structural studies and electrocatalytic water oxidation properties of molecular copper complexes: The influence of N-alkylation of ligand and coordination number
In this study, we report the synthesis, structural studies, and electrocatalytic water oxidation properties of three novel Cu complexes featuring 2-pyridylmethyl-substituted 1,4,7-triazacyclononane ligands. Structural characterizations reveal the penta-coordination structure of complex [Cu(Py2tcan)](ClO4)2 (1, Py2tcan = 1,4-bis(2-picolyl)-1,4,7-triazacyclononane) and complex [Cu(MePy2tcan)](ClO4)2 (2, MePy2tcan = 1-methyl-4,7-bis(2-picolyl)-1,4,7-triazacyclononane), as well as the six-coordination structure of complex [Cu(Py3tcan)](ClO4)2 (3, Py3tcan = 1,4,7-tris(2-picolyl)-1,4,7-triazacyclononane). Systematic electrochemical analysis reveals that 1 and 2 exhibit high catalytic efficiency for water oxidation. Mechanistic studies suggest that the secondary amine coordination in 1 significantly reduces the catalytic overpotential by enhancing the σ-donor effect, thereby facilitating Cu center oxidation and enhancing the catalytic cycle. While 2 featuring two pyridine groups and three tertiary amine groups in its coordination environment performs higher catalytic overpotential than 1 because of the poor σ-donor properties of its N–CH3 structure. Besides, 3 shows much lower catalytic activity, underscoring the necessity of an unsaturated coordination geometry for efficient water oxidation. Compared to its analogous Ni complex [Ni(Py3tcan)]2+ (4) and Fe complex [Fe(Py3tcan)]2+, 3 shows higher onset overpotential and lower catalytic activity, indicating that even when stabilized by the same Py3tacn ligand, the Cu center possesses inferior intrinsic catalytic activity for water oxidation relative to Ni and Fe centers. Nevertheless, the catalytic performance of such Cu complex can be enhanced through rational modulation of the ligand structure and coordination number.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Molecular Structure
化学-物理化学
CiteScore
7.10
自引率
15.80%
发文量
2384
审稿时长
45 days
期刊介绍:
The Journal of Molecular Structure is dedicated to the publication of full-length articles and review papers, providing important new structural information on all types of chemical species including:
• Stable and unstable molecules in all types of environments (vapour, molecular beam, liquid, solution, liquid crystal, solid state, matrix-isolated, surface-absorbed etc.)
• Chemical intermediates
• Molecules in excited states
• Biological molecules
• Polymers.
The methods used may include any combination of spectroscopic and non-spectroscopic techniques, for example:
• Infrared spectroscopy (mid, far, near)
• Raman spectroscopy and non-linear Raman methods (CARS, etc.)
• Electronic absorption spectroscopy
• Optical rotatory dispersion and circular dichroism
• Fluorescence and phosphorescence techniques
• Electron spectroscopies (PES, XPS), EXAFS, etc.
• Microwave spectroscopy
• Electron diffraction
• NMR and ESR spectroscopies
• Mössbauer spectroscopy
• X-ray crystallography
• Charge Density Analyses
• Computational Studies (supplementing experimental methods)
We encourage publications combining theoretical and experimental approaches. The structural insights gained by the studies should be correlated with the properties, activity and/ or reactivity of the molecule under investigation and the relevance of this molecule and its implications should be discussed.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


