一种对称化学传感器合成苯并呋喃-2-羧酰胺衍生物的新方法,用于CN -离子基偶氮噻唑烷-2,4 -二酮(S-AT)的裸眼检测、抗菌活性评价和分子对接研究
IF 4.7
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
合成了一种新的含噻唑烷-2,4-dions (TZD)和偶氮发色团的对称化学传感器,并将其用作比色传感器。检测阴离子的目视研究表明,该传感器是氰化物离子(CN−)的高灵敏度和选择性显色检测器。CN -可观察到的颜色从黄色到橙色的变化使得不需要使用先进的设备,甚至用肉眼也能检测到这种离子。传感器对CN−离子的检出限(LOD)为0.145 μM。Job图表明,传感器(S-AT)与CN -的化学计量比为1:2。这种传感器可以用于现实世界,包括环境监测和化学分析,因为它能够快速准确地检测到危险的CN - s。此外,S-AT对革兰氏阳性菌(金黄色葡萄球菌)也显示出显著的效果。试图澄清用CN -处理S-AT的结果,以了解事件序列导致反应物颜色变化的机制。计算表明,在这种情况下,CN -的碱度比其亲核性占优势,并导致了一个新的途径,在这个途径中,5个原子的6π电环闭合发生,然后是羰基硫分子的挤压,最后提供苯并呋喃-2-羧酰胺部分,其存在与实验FT-IR, UV-Vis光谱和1H NMR相容。通过对接研究,探索了一些相关拟结构治疗癌症和阿尔茨海默病的有效性,并取得了显著成果。本文章由计算机程序翻译,如有差异,请以英文原文为准。
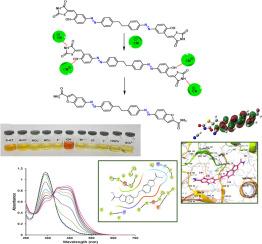
A new method for synthesizing benzofuran-2-carboxamide derivatives with a symmetrical chemosensor for naked-eye detection of CN– ions based azo-thiazolidine-2,4‑dione (S-AT) and evaluation of antibacterial activity and molecular docking studies
A new symmetric chemosensor including a thiazolidine-2,4-dions (TZD) and an azo chromophore was synthesized and employed as a colorimetric sensor. Visual investigations of detecting anions showed that the sensor acts as a highly sensitive and selective chromogenic detector of cyanide ions (CN−). The observable color change from Yellow to Orange for CN− allows the detection of this ion without the use of advanced equipment, even with the naked eye. The sensor limit of detection (LOD) was determined to be 0.145 μM for CN− ions. The Job plot indicates that the sensor (S-AT) binds to CN− with a stoichiometric ratio of 1:2. This sensor can be used in the real world, including environmental monitoring and chemical analysis, because of its ability to quickly and accurately detect dangerous CN−s. Also, S-AT against Gram-positive bacteria (S. aureus) shows a significant result. An attempt has been made to clarify the results of the treatment of S-AT with CN− to understand the mechanism in which the sequence of events leads to a change in the color of the reactant. It has been demonstrated computationally that in this case the basicity of CN− is dominant over its nucleophilicity and causes proceeding a new route in which a five atoms 6π electrocyclic ring closure occurs that is followed by extrusion of carbonyl sulfide molecules and finally affords benzofuran-2-carboxamide moiety whose presence is compatible with the experimental FT-IR, UV–Vis spectra, and 1H NMR. The effectiveness of some related proposed structures for treating Cancer and Alzheimer’s disease were explored through docking studies, and significant results were obtained.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Molecular Structure
化学-物理化学
CiteScore
7.10
自引率
15.80%
发文量
2384
审稿时长
45 days
期刊介绍:
The Journal of Molecular Structure is dedicated to the publication of full-length articles and review papers, providing important new structural information on all types of chemical species including:
• Stable and unstable molecules in all types of environments (vapour, molecular beam, liquid, solution, liquid crystal, solid state, matrix-isolated, surface-absorbed etc.)
• Chemical intermediates
• Molecules in excited states
• Biological molecules
• Polymers.
The methods used may include any combination of spectroscopic and non-spectroscopic techniques, for example:
• Infrared spectroscopy (mid, far, near)
• Raman spectroscopy and non-linear Raman methods (CARS, etc.)
• Electronic absorption spectroscopy
• Optical rotatory dispersion and circular dichroism
• Fluorescence and phosphorescence techniques
• Electron spectroscopies (PES, XPS), EXAFS, etc.
• Microwave spectroscopy
• Electron diffraction
• NMR and ESR spectroscopies
• Mössbauer spectroscopy
• X-ray crystallography
• Charge Density Analyses
• Computational Studies (supplementing experimental methods)
We encourage publications combining theoretical and experimental approaches. The structural insights gained by the studies should be correlated with the properties, activity and/ or reactivity of the molecule under investigation and the relevance of this molecule and its implications should be discussed.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


