新型噻唑啉衍生物:合成、表征和潜在抗癌药物的分子对接研究
IF 4.7
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
合成新的噻唑啉衍生物,并与标准药物5-氟尿嘧啶比较,评价其结构特征和分子结合亲和力。以等摩尔量的二硫化碳和3-氯乙酰-2,4 -二酮与芳香胺衍生物在无水乙醇中反应,合成了新的噻唑啉衍生物。合成化合物的熔点用电熔点仪测定。利用傅里叶变换红外光谱(FTIR-8400)、质子核磁共振(1HNMR)、碳-13核磁共振(13CNMR)和气相色谱-质谱(GC-MS) (MASS-ll模型)进行结构表征。使用AutoDock Vina进行分子对接研究,以评估合成化合物与选定蛋白靶点之间的结合相互作用,并与5-氟尿嘧啶的结合亲和力进行比较。合成的噻唑啉衍生物具有明显的熔点,并通过应用光谱技术成功地进行了表征。分子对接结果显示,合成的化合物与靶蛋白的自由结合能高于5-氟尿嘧啶,表明蛋白质与配体的相互作用可能更强。新合成的噻唑啉衍生物与靶蛋白的结合亲和力较好,超过了传统药物5-氟尿嘧啶,因此可能成为进一步药物开发的潜在候选药物。本文章由计算机程序翻译,如有差异,请以英文原文为准。
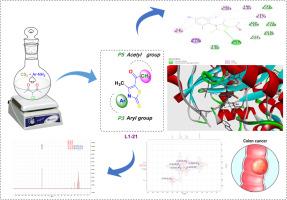
Novel thiazoline derivatives: Synthesis, characterisation, and molecular docking investigations as potential anticancer agents
To synthesise new thiazoline derivative compounds and evaluate their structural characteristics and molecular binding affinities in comparison to the standard drug 5-fluorouracil.
New thiazoline derivatives were synthesised by reacting equimolar amounts of carbon disulfide and 3-chloroacetyl-2,4‑dione with aromatic amine derivatives in absolute ethanol. The melting points of the synthesised compounds were determined using an Electro Melting Point Apparatus. Structural characterisation was carried out utilizing Fourier-transform infrared spectroscopy (FTIR-8400), proton nuclear magnetic resonance (1HNMR ),carbon-13 nuclear magnetic resonance (13CNMR ), and gas chromatography-mass spectrometry GC–MS, model MASS-ll. Molecular docking studies were performed using AutoDock Vina to assess the binding interactions between the synthesised compounds and selected protein targets, with comparisons made to the binding affinity of 5-fluorouracil. The synthesised thiazoline derivatives exhibited distinct melting points and were successfully characterised by the applied spectroscopic techniques. Molecular docking results revealed that the free binding energies of the synthesised compounds with the target proteins were higher than those of 5-fluorouracil, suggesting potentially stronger protein-ligand interactions. The newly synthesised thiazoline derivatives demonstrate promising binding affinities towards target proteins, surpassing those of the conventional drug 5-fluorouracil, and thus may serve as potential candidates for further pharmaceutical development.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Molecular Structure
化学-物理化学
CiteScore
7.10
自引率
15.80%
发文量
2384
审稿时长
45 days
期刊介绍:
The Journal of Molecular Structure is dedicated to the publication of full-length articles and review papers, providing important new structural information on all types of chemical species including:
• Stable and unstable molecules in all types of environments (vapour, molecular beam, liquid, solution, liquid crystal, solid state, matrix-isolated, surface-absorbed etc.)
• Chemical intermediates
• Molecules in excited states
• Biological molecules
• Polymers.
The methods used may include any combination of spectroscopic and non-spectroscopic techniques, for example:
• Infrared spectroscopy (mid, far, near)
• Raman spectroscopy and non-linear Raman methods (CARS, etc.)
• Electronic absorption spectroscopy
• Optical rotatory dispersion and circular dichroism
• Fluorescence and phosphorescence techniques
• Electron spectroscopies (PES, XPS), EXAFS, etc.
• Microwave spectroscopy
• Electron diffraction
• NMR and ESR spectroscopies
• Mössbauer spectroscopy
• X-ray crystallography
• Charge Density Analyses
• Computational Studies (supplementing experimental methods)
We encourage publications combining theoretical and experimental approaches. The structural insights gained by the studies should be correlated with the properties, activity and/ or reactivity of the molecule under investigation and the relevance of this molecule and its implications should be discussed.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


