嘧啶-2,4-二氯苯偶联物作为痛风治疗黄嘌呤氧化酶拮抗剂的开发:计算和实验研究
IF 4.7
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
设计、合成了一种新型的含有2,4-二氯苯基核心的嘧啶基杂合体,并对其作为治疗痛风性关节炎的潜在黄嘌呤氧化酶(XO)抑制剂进行了评估。通过既定的药物化学合成方法,成功制备了这些化合物,并通过详细的光谱分析,包括高分辨率质谱(HRMS),傅里叶变换红外(FT-IR)和多核磁共振(¹H和¹³C)证实了它们的结构。在体外筛选中,9个候选蛋白(2、3、9、10、11a、11d、11e、12a和12b)评估了它们的XO抑制活性、防止蛋白变性的能力和稳定红细胞膜的能力。值得注意的是,化合物12b成为最有希望的药剂,具有卓越的XO抑制作用(IC₅₀= 2.8 μM),有效的蛋白质变性抑制作用(100 μg/mL时为89.4%),并显着降低溶血(92.1%功效),超过别嘌呤醇和双氯芬酸等标准药物。分子对接模拟表明,化合物与XO活性位点之间存在强结合,其中12b通过氢键和疏水接触表现出最佳相互作用。这些计算结果与实验结果一致,提示竞争性抑制的机制。总的来说,这些发现突出了12b作为痛风治疗的主要候选药物的治疗潜力,为进一步优化开发具有更高疗效和选择性的高级XO抑制剂提供了基础。本文章由计算机程序翻译,如有差异,请以英文原文为准。
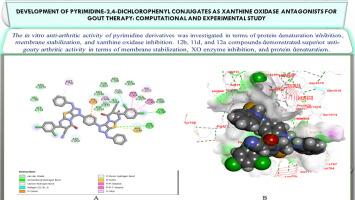
Development of Pyrimidine-2,4-Dichlorophenyl Conjugates as Xanthine Oxidase Antagonists for Gout Therapy: Computational and Experimental Study
A novel group of pyrimidine-based hybrids containing a 2,4-dichlorophenyl core was designed, synthesized, and evaluated as potential xanthine oxidase (XO) inhibitors for treating gouty arthritis. Through established medicinal chemistry synthetic methods, these compounds were successfully prepared and their structures confirmed using detailed spectroscopic analyses, including high-resolution mass spectrometry (HRMS), Fourier-transform infrared (FT-IR), and multinuclear NMR (¹H and ¹³C). In vitro screening, nine candidates (2, 3, 9, 10, 11a, 11d, 11e, 12a, and 12b) assessed their XO inhibitory activity, ability to prevent protein denaturation, and capability to stabilize erythrocyte membranes. Notably, compound 12b emerged as the most promising agent, displaying superior XO inhibition (IC₅₀ = 2.8 μM), effective protein denaturation suppression (89.4% at 100 μg/mL), and significant reduction in hemolysis (92.1% efficacy), surpassing standard drugs like allopurinol and diclofenac. Molecular docking simulations indicated strong binding between the compounds and the XO active site, with 12b showing optimal interactions through hydrogen bonding and hydrophobic contacts. These computational results were consistent with experimental outcomes, suggesting a mechanism of competitive inhibition. The overall findings highlight the therapeutic potential of 12b as a leading candidate for gout management, providing a foundation for further optimization in developing advanced XO inhibitors with improved efficacy and selectivity.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Molecular Structure
化学-物理化学
CiteScore
7.10
自引率
15.80%
发文量
2384
审稿时长
45 days
期刊介绍:
The Journal of Molecular Structure is dedicated to the publication of full-length articles and review papers, providing important new structural information on all types of chemical species including:
• Stable and unstable molecules in all types of environments (vapour, molecular beam, liquid, solution, liquid crystal, solid state, matrix-isolated, surface-absorbed etc.)
• Chemical intermediates
• Molecules in excited states
• Biological molecules
• Polymers.
The methods used may include any combination of spectroscopic and non-spectroscopic techniques, for example:
• Infrared spectroscopy (mid, far, near)
• Raman spectroscopy and non-linear Raman methods (CARS, etc.)
• Electronic absorption spectroscopy
• Optical rotatory dispersion and circular dichroism
• Fluorescence and phosphorescence techniques
• Electron spectroscopies (PES, XPS), EXAFS, etc.
• Microwave spectroscopy
• Electron diffraction
• NMR and ESR spectroscopies
• Mössbauer spectroscopy
• X-ray crystallography
• Charge Density Analyses
• Computational Studies (supplementing experimental methods)
We encourage publications combining theoretical and experimental approaches. The structural insights gained by the studies should be correlated with the properties, activity and/ or reactivity of the molecule under investigation and the relevance of this molecule and its implications should be discussed.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


