2,6-二吡嗪基吡啶的结构表征、抗癌特性和BSA结合:来自实验和理论的见解
IF 4.7
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
以2-乙酰吡嗪和取代苯甲醛为原料,采用一锅式krohnke法合成了苯基(2,6-二-2-吡嗪基)吡啶衍生物(L1, L2)。利用核磁共振、HRMS和单晶x射线衍射技术,结合密度泛函理论(DFT)对其结构进行了表征。单晶x射线衍射表明,L1在C2/c空间群(T = 296 K)中结晶,其超分子组装被c - h⋯N和π -π堆叠相互作用稳定,而L2促进c - h⋯N, N - h⋯π分叉和π -π *相互作用。以牛血清白蛋白(BSA)为模型蛋白,采用荧光光谱法研究L1的生物相互作用特性。荧光研究表明L1诱导BSA的静态猝灭,其结合常数为5.15 × 10⁴mol·dm⁻³。同步荧光光谱和三维荧光光谱进一步表明,L1引起了牛血清白蛋白明显的构象变化。评价了化合物对HCT-116人结直肠癌细胞系的细胞毒性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
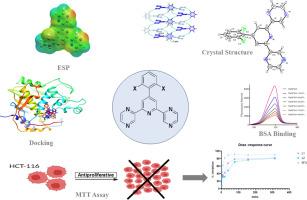
Structural characterisation, anticancer properties, and BSA binding of 2,6-dipyrazinylpyridines: Insights from experiment and theory
The phenyl-(2,6-di-2-pyrazinyl)pyridine derivatives (L1, L2) were synthesized using a one-pot Krohnke-type method, starting from 2-acetylpyrazine and substituted benzaldehydes. Their structures were characterized using a combination of spectroscopic (NMR, HRMS) and single-crystal X-ray diffraction techniques, complemented by density functional theory (DFT). Single-crystal X-ray diffraction reveals that L1 crystallizes in the C2/c space group (T = 296 K) with its supramolecular assembly being stabilized by C–H⋯N and π–π stacking interactions, whereas L2 facilitates C–H⋯N, N–H⋯π bifurcated, and π–π* interactions. The bio-interaction properties of L1 were studied using fluorescence spectroscopy with bovine serum albumin (BSA) as a model protein. Fluorescence studies demonstrated L1 induces static quenching of BSA, with a binding constant of 5.15 × 10⁴ mol·dm⁻³. Synchronous and three-dimensional fluorescence spectra further demonstrated that L1 brings forth significant conformational changes in BSA. The compounds were evaluated for cytotoxicity against the HCT-116 human colorectal cancer cell line.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Molecular Structure
化学-物理化学
CiteScore
7.10
自引率
15.80%
发文量
2384
审稿时长
45 days
期刊介绍:
The Journal of Molecular Structure is dedicated to the publication of full-length articles and review papers, providing important new structural information on all types of chemical species including:
• Stable and unstable molecules in all types of environments (vapour, molecular beam, liquid, solution, liquid crystal, solid state, matrix-isolated, surface-absorbed etc.)
• Chemical intermediates
• Molecules in excited states
• Biological molecules
• Polymers.
The methods used may include any combination of spectroscopic and non-spectroscopic techniques, for example:
• Infrared spectroscopy (mid, far, near)
• Raman spectroscopy and non-linear Raman methods (CARS, etc.)
• Electronic absorption spectroscopy
• Optical rotatory dispersion and circular dichroism
• Fluorescence and phosphorescence techniques
• Electron spectroscopies (PES, XPS), EXAFS, etc.
• Microwave spectroscopy
• Electron diffraction
• NMR and ESR spectroscopies
• Mössbauer spectroscopy
• X-ray crystallography
• Charge Density Analyses
• Computational Studies (supplementing experimental methods)
We encourage publications combining theoretical and experimental approaches. The structural insights gained by the studies should be correlated with the properties, activity and/ or reactivity of the molecule under investigation and the relevance of this molecule and its implications should be discussed.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


