利用多光谱、分子对接和分子动力学研究余甘子鞣花单宁与乙酰胆碱酯酶的相互作用机制
IF 4.7
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
鞣花丹宁是广泛存在于余甘子中的一类可水解单宁,对乙酰胆碱酯酶(AChE)活性具有抑制作用;然而,迄今为止,它们的抑制机制仍不清楚。本研究采用酶抑制动力学、多光谱分析、分子对接、分子动力学模拟等方法,系统分析了四种鞣花单宁对AChE的抑制机制。酶抑制动力学表明,四种鞣花单宁都是可逆的乙酰胆碱酯酶抑制剂,其中鞣花单宁是一种混合抑制剂,鞣花酸、天竺葵苷和天竺葵酸是一种非竞争性抑制剂。荧光光谱证实四种鞣花单宁与AChE形成配合物,通过静态猝灭使AChE的固有荧光猝灭,只有一个结合位点。结合主要由氢键和范德华力驱动,结合过程为焓驱动的自发放热过程。它还能猝灭酪氨酸(Tyr)和色氨酸(Trp)的同步荧光,改变色氨酸残基周围的微环境。荧光共振能量传递进一步验证了荧光猝灭是非辐射能量共振和静态猝灭的结果。紫外可见光谱、红外光谱和CD光谱结果表明,酶抑制剂复合物的形成改变了乙酰胆碱酯酶的二级结构。分子对接和分子动力学模拟进一步验证了四种鞣花单宁嵌入AChE疏水口袋形成稳定的配合物,并与周围氨基酸残基形成氢键,使酶结构更加稳定,阻碍底物进入,最终抑制AChE活性。总的来说,我们的研究为鞣花单宁作为天然乙酰胆碱酯酶抑制剂及其作为阿尔茨海默病治疗候选药物的潜力提供了新的见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
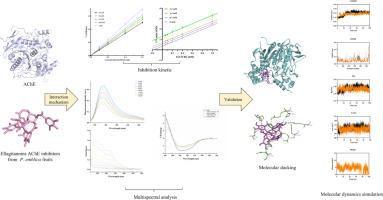
Study of the interaction mechanisms of ellagitannins from Phyllanthus emblica L. with acetylcholinesterase using multi-spectroscopy, molecular docking, and molecular dynamic
Ellagitannins, a class of hydrolysable tannins widely present in Phyllanthus emblica L., exhibit inhibitory effects on acetylcholinesterase (AChE) activity; however, their inhibition mechanisms remain poorly characterized to date. In this study, the inhibition mechanism between four ellagitannins and AChE was systematically analyzed by integrating enzyme inhibition kinetics, multi-spectral analysis, molecular docking and molecular dynamics simulation. Enzyme inhibition kinetics indicated that all four ellagitannins were reversible inhibitors of AChE, with corilagin being a mixed inhibitor and ellagic acid, geraniin and chebulagic acid being uncompetitive inhibitors. Fluorescence spectroscopy confirmed that the four ellagitannins formed complexes with AChE and quenched the intrinsic fluorescence of AChE through static quenching, with only one binding site. The binding was mainly driven by hydrogen bonds and van der Waals forces, and the binding process was an enthalpy-driven spontaneous exothermic process. It can also quench the synchronous fluorescence of tyrosine (Tyr) and tryptophan (Trp), and change the microenvironment around the tryptophan residues. Fluorescence resonance energy transfer further verified that the fluorescence quenching was the result of non-radiative energy resonance and static quenching. UV–vis, FT-IR and CD spectroscopy results showed that the formation of enzyme-inhibitor complexes altered the secondary structure of AChE. Molecular docking and molecular dynamics simulation further verified that the four ellagitannins embedded in the hydrophobic pocket of AChE to form stable complexes, and formed hydrogen bonds with surrounding amino acid residues, making the enzyme structure more stable and hindering the entry of substrates, ultimately inhibiting AChE activity. Overall, our study provides novel insights into ellagitannins as natural AChE inhibitors and their potential as therapeutic candidates for Alzheimer’s disease.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Molecular Structure
化学-物理化学
CiteScore
7.10
自引率
15.80%
发文量
2384
审稿时长
45 days
期刊介绍:
The Journal of Molecular Structure is dedicated to the publication of full-length articles and review papers, providing important new structural information on all types of chemical species including:
• Stable and unstable molecules in all types of environments (vapour, molecular beam, liquid, solution, liquid crystal, solid state, matrix-isolated, surface-absorbed etc.)
• Chemical intermediates
• Molecules in excited states
• Biological molecules
• Polymers.
The methods used may include any combination of spectroscopic and non-spectroscopic techniques, for example:
• Infrared spectroscopy (mid, far, near)
• Raman spectroscopy and non-linear Raman methods (CARS, etc.)
• Electronic absorption spectroscopy
• Optical rotatory dispersion and circular dichroism
• Fluorescence and phosphorescence techniques
• Electron spectroscopies (PES, XPS), EXAFS, etc.
• Microwave spectroscopy
• Electron diffraction
• NMR and ESR spectroscopies
• Mössbauer spectroscopy
• X-ray crystallography
• Charge Density Analyses
• Computational Studies (supplementing experimental methods)
We encourage publications combining theoretical and experimental approaches. The structural insights gained by the studies should be correlated with the properties, activity and/ or reactivity of the molecule under investigation and the relevance of this molecule and its implications should be discussed.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


