作为降糖药的1,3,4-噻二唑衍生物:设计、合成、生物学评价和硅研究
IF 4.7
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
本文报道了12种1,3,4-噻二唑衍生物(9a-9l)的设计、合成和评价。筛选所得化合物对α-淀粉酶和α-葡萄糖苷酶具有抑制作用,其中部分化合物表现出中强抑制作用。值得注意的是,化合物9e、9f和9i表现出最有效的作用,IC₅₀值分别为19.25±0.183 μM、18.71±0.015 μM和18.78±0.136 μM,与标准抑制剂相当。分子对接研究强调了α-葡萄糖苷酶和α-淀粉酶关键催化残基之间稳定的氢键和疏水相互作用,支持了实验结果。构效关系(SAR)分析表明卤素和烷基取代对药效的增强有重要作用。虽然结果表明噻二唑支架有望作为抗高血糖先导物,但进一步的优化、ADMET分析和体内验证将对这些衍生物的治疗发展至关重要。本文章由计算机程序翻译,如有差异,请以英文原文为准。
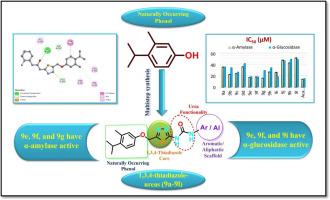
1,3,4-thiadiazole derivatives as antidiabetic agents: Design, synthesis, biological evaluation, and in silico studies
The present work reports the design, synthesis, and evaluation of twelve 1,3,4-thiadiazole derivatives (9a–9l) as potential antidiabetic agents. The compounds were screened for α-amylase and α-glucosidase inhibition, with several showing moderate to strong activity. Notably, compounds 9e, 9f, and 9i exhibited the most potent effects with IC₅₀ values of 19.25 ± 0.183 μM, 18.71 ± 0.015 μM, and 18.78 ± 0.136 μM, respectively, comparable to standard inhibitors. Molecular docking studies supported the experimental findings by highlighting stabilizing hydrogen bonding and hydrophobic interactions with key catalytic residues of α-glucosidase and α-amylase. Structure–activity relationship (SAR) analysis suggested the role of halogen and alkyl substitutions in enhance potency. While the results indicate that thiadiazole scaffolds hold promise as anti-hyperglycemic leads, further optimization, ADMET profiling, and in vivo validation will be essential advance these derivatives toward therapeutic development.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Molecular Structure
化学-物理化学
CiteScore
7.10
自引率
15.80%
发文量
2384
审稿时长
45 days
期刊介绍:
The Journal of Molecular Structure is dedicated to the publication of full-length articles and review papers, providing important new structural information on all types of chemical species including:
• Stable and unstable molecules in all types of environments (vapour, molecular beam, liquid, solution, liquid crystal, solid state, matrix-isolated, surface-absorbed etc.)
• Chemical intermediates
• Molecules in excited states
• Biological molecules
• Polymers.
The methods used may include any combination of spectroscopic and non-spectroscopic techniques, for example:
• Infrared spectroscopy (mid, far, near)
• Raman spectroscopy and non-linear Raman methods (CARS, etc.)
• Electronic absorption spectroscopy
• Optical rotatory dispersion and circular dichroism
• Fluorescence and phosphorescence techniques
• Electron spectroscopies (PES, XPS), EXAFS, etc.
• Microwave spectroscopy
• Electron diffraction
• NMR and ESR spectroscopies
• Mössbauer spectroscopy
• X-ray crystallography
• Charge Density Analyses
• Computational Studies (supplementing experimental methods)
We encourage publications combining theoretical and experimental approaches. The structural insights gained by the studies should be correlated with the properties, activity and/ or reactivity of the molecule under investigation and the relevance of this molecule and its implications should be discussed.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


