新型硫醚衍生物的设计、合成及其杀虫活性
IF 4.7
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
蚊子是疾病的重要传播媒介,每年造成数万人死亡,并给受影响地区带来沉重的治疗费用。虽然杀虫剂在控制有害生物损害方面是有效的,但它们的广泛和持续使用已导致抗药性的出现,对有害生物防治提出了重大挑战。为了解决这个问题,新型杀虫剂的设计至关重要。合成了一系列新型硫醚化合物,并采用支架跳跃法对其进行了表征,并对其对信天翁阿蚊幼虫和成蚊,以及对芸苔短尾蛾和油菜小Pieris的杀虫活性进行了评价。所有目标化合物均表现出优异的杀虫活性。通过Ellman方法和分子对接研究对其杀虫机理进行了研究。此外,利用DFT计算分析了化合物A7 (LC50=0.0155 mg/L)的杀虫活性低于阳性对照氯氰菊酯(LC50=0.0145 mg/L),而高于敌敌畏(LC50=0.0469 mg/L)的原因。建立了3D-QSAR模型来预测这类化合物的功效,其ADME特性显示出良好的亲脂性和良好的口服生物利用度。这项研究的结果预计将有助于开发更有效和环境友好的农用化学品,解决害虫抗性问题。本文章由计算机程序翻译,如有差异,请以英文原文为准。
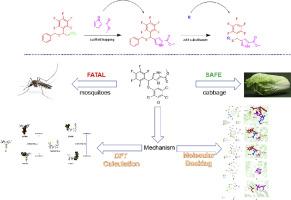
Design, synthesis, and insecticidal activities of novel thioether derivatives
Mosquitoes are significant vectors of diseases which cause tens of thousands of deaths annually and impose heavy treatment costs on affected regions. Although insecticides have been effective in managing pest damage, their widespread and continuous use has led to the emergence of resistance, presenting a major challenge in pest control. To address this issue, the design of novel insecticides is crucial. A series of novel thioether compounds were synthesized and characterized via a scaffold hopping strategy and their insecticidal activity against both larvae and adult mosquitoes of Armigeres subalbatus, as well as against Brevicoryne brassicae and Pieris rapae Linn were evaluated. All target compounds exhibited excellent insecticidal activity. The insecticidal mechanisms were investigated through Ellman’s method and molecular docking studies. Additionally, DFT calculations were used to analyze why compound A7 (LC50=0.0155 mg/L) showed less insecticidal activity compared to the positive control, cypermethrin (LC50=0.0145 mg/L) and higher insecticidal activity compared to dichlorvos (LC50=0.0469 mg/L). A 3D-QSAR model was developed to predict the efficacy of this class of compounds and the ADME properties revealed good lipophilicity and well-predicted oral bioavailability. The findings of this study are expected to contribute to the development of more effective and environmentally friendly agricultural chemicals, addressing the issue of pest resistance.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Molecular Structure
化学-物理化学
CiteScore
7.10
自引率
15.80%
发文量
2384
审稿时长
45 days
期刊介绍:
The Journal of Molecular Structure is dedicated to the publication of full-length articles and review papers, providing important new structural information on all types of chemical species including:
• Stable and unstable molecules in all types of environments (vapour, molecular beam, liquid, solution, liquid crystal, solid state, matrix-isolated, surface-absorbed etc.)
• Chemical intermediates
• Molecules in excited states
• Biological molecules
• Polymers.
The methods used may include any combination of spectroscopic and non-spectroscopic techniques, for example:
• Infrared spectroscopy (mid, far, near)
• Raman spectroscopy and non-linear Raman methods (CARS, etc.)
• Electronic absorption spectroscopy
• Optical rotatory dispersion and circular dichroism
• Fluorescence and phosphorescence techniques
• Electron spectroscopies (PES, XPS), EXAFS, etc.
• Microwave spectroscopy
• Electron diffraction
• NMR and ESR spectroscopies
• Mössbauer spectroscopy
• X-ray crystallography
• Charge Density Analyses
• Computational Studies (supplementing experimental methods)
We encourage publications combining theoretical and experimental approaches. The structural insights gained by the studies should be correlated with the properties, activity and/ or reactivity of the molecule under investigation and the relevance of this molecule and its implications should be discussed.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


