Bis-Schiff碱作为有效的抗糖尿病药物:合成、酶抑制、分子对接和动态模拟
IF 4.7
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
双希夫碱具有α-淀粉酶和α-葡萄糖苷酶的抑制作用,是药物化学中重要的化合物。合成了一系列4-羟基苯乙酮双希夫碱,对其体外α-淀粉酶和α-葡萄糖苷酶抑制活性进行了表征和测试。其中,7个化合物(2d、2c、2q、2i、2h、2a和2g)的IC50值为α-淀粉酶的IC50 = 3.95±0.09,α-葡萄糖苷酶的IC50 = 4.33±0.07µM, α-葡萄糖苷酶的IC50 = 19.64±0.04,α-葡萄糖苷酶的IC50 = 21.53±0.05µM,具有较好的抑制效果。与标准阿卡波糖相比,5个化合物表现出中等活性,其余5个化合物表现出较低的抑制活性。构效关系(SAR)研究表明,给电子基团(甲氧基和羟基)的存在增加了化合物的抑制活性。与α-淀粉酶的分子对接研究表明,合成序列的对接得分与IC50值之间存在合理的相关性,验证了对接方法对这些类似物进行排序的有效性。这项工作强调了合成双希夫碱作为抗糖尿病药物的希望,基于其有效的α-淀粉酶和α-葡萄糖苷酶抑制。本文章由计算机程序翻译,如有差异,请以英文原文为准。
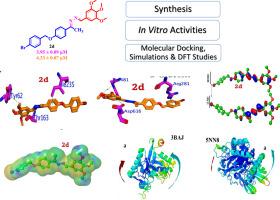
Bis-Schiff bases as potent antidiabetic agents: Synthesis, enzyme inhibition, molecular docking and dynamic simulations
Bis-Schiff bases are significant compounds in medicinal chemistry due to the inhibition of α-amylase and α-glucosidase enzymes. A series of bis-Schiff bases of 4-hydroxyacetophenone were synthesized, characterized and tested for their in vitro α-amylase and α-glucosidase inhibitory activities. Among the series, seven compounds (2d, 2c, 2q, 2i, 2h, 2a, and 2g) attributed excellent inhibitory effect with IC50 values ranging from (IC50 = 3.95 ± 0.09 for α-amylase and 4.33 ± 0.07 µM for α-glucosidase) to (IC50 = 19.64 ± 0.04 for α-amylase and 21.53 ± 0.05 µM for α-glucosidase). Similarly, five compounds were found moderate active while the remaining five compounds showed less inhibitory activities by comparing with the standard acarbose. The structure activity relationship (SAR) study showed that the existence of electron donating groups (methoxy and hydroxyl) increases the inhibitory activities of the compounds. The molecular docking study against α-amylase, revealed a reasonable correlation between docking scores and IC50 values was observed for the synthesized series, validating the docking approach for ranking these analogues. This work highpoints the synthetic bis-Schiff bases as hopeful antidiabetic agents based on their potent α-amylase and α-glucosidase inhibition.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Molecular Structure
化学-物理化学
CiteScore
7.10
自引率
15.80%
发文量
2384
审稿时长
45 days
期刊介绍:
The Journal of Molecular Structure is dedicated to the publication of full-length articles and review papers, providing important new structural information on all types of chemical species including:
• Stable and unstable molecules in all types of environments (vapour, molecular beam, liquid, solution, liquid crystal, solid state, matrix-isolated, surface-absorbed etc.)
• Chemical intermediates
• Molecules in excited states
• Biological molecules
• Polymers.
The methods used may include any combination of spectroscopic and non-spectroscopic techniques, for example:
• Infrared spectroscopy (mid, far, near)
• Raman spectroscopy and non-linear Raman methods (CARS, etc.)
• Electronic absorption spectroscopy
• Optical rotatory dispersion and circular dichroism
• Fluorescence and phosphorescence techniques
• Electron spectroscopies (PES, XPS), EXAFS, etc.
• Microwave spectroscopy
• Electron diffraction
• NMR and ESR spectroscopies
• Mössbauer spectroscopy
• X-ray crystallography
• Charge Density Analyses
• Computational Studies (supplementing experimental methods)
We encourage publications combining theoretical and experimental approaches. The structural insights gained by the studies should be correlated with the properties, activity and/ or reactivity of the molecule under investigation and the relevance of this molecule and its implications should be discussed.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


