Wnt/β-catenin信号以代谢底物依赖的方式调节心脏Cx43
IF 2.2
Journal of molecular and cellular cardiology plus
Pub Date : 2025-10-02
DOI:10.1016/j.jmccpl.2025.100488
引用次数: 0
摘要
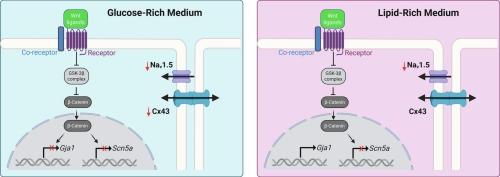
背景Nav1.5和Cx43对心肌快速电脉冲传导至关重要,它们的减少产生了致心律失常的底物。Wnt/β-catenin信号在致心律失常心肌中被激活,尽管已知该信号下调心脏Nav1.5,但其对Cx43的调控尚不清楚,报道的结果相互矛盾。本研究调查了Wnt/β-catenin信号如何调节大鼠和人心肌细胞中的Cx43,以及它是否依赖于细胞的性别或代谢底物。方法用CHIR-99021 (CHIR)或Wnt3a蛋白(两种不同的Wnt/β-catenin信号激活剂)处理雄性和雌性新生大鼠心室肌细胞(nrvm),在富含葡萄糖(心力衰竭的首选代谢底物)或富含脂质(~ 150 μM脂肪酸,健康心脏的首选底物)的培养基中处理。使用健康和Brugada综合征人ipsc来源的心肌细胞(iPSC-CMs)来证实nrvm中的观察结果。结果在富糖培养基中,Gja1 mRNA(编码Cx43)在雌性nrvm中被低浓度CHIR (1 μM)还原,而在雄性nrvm中只被高浓度CHIR (10 μM)还原。然而,在雄性和雌性nrvm中,在1 μM CHIR时都观察到Cx43蛋白的减少,这表明转录和转录后机制都参与其中。在富脂培养基中,CHIR在1 μM或3 μM下均未改变Gja1 mRNA和Cx43蛋白。相比之下,在富含葡萄糖和富含脂质的培养基中,chir诱导的Scn5a mRNA和Nav1.5蛋白的减少均被观察到,没有发现显著的性别特异性差异。与使用与Wnt受体无关的激活剂CHIR的研究一致,Wnt3a蛋白在富葡萄糖培养基中也降低了nrvm中的Gja1 mRNA和Cx43蛋白,而在富脂培养基中则没有。在两名健康志愿者和一名Brugada综合征患者的人类iPSC-CMs中,Wnt/β-catenin信号激活在标准的含葡萄糖培养基中降低了GJA1 mRNA和Cx43蛋白。这些数据表明,代谢底物调节心肌细胞中Wnt/β-catenin信号的作用,仅当葡萄糖是主要代谢底物时才观察到Cx43 mRNA和蛋白的减少,这种情况发生在心律失常的情况下,如心脏肥厚和心力衰竭。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Wnt/β-catenin signaling regulates cardiac Cx43 in a metabolic substrate-dependent manner
Background
Both Nav1.5 and Cx43 are critical for the fast electrical impulse conduction in the myocardium and their reductions create the arrhythmogenic substrate. Wnt/β-catenin signaling is activated in arrhythmogenic myocardium, and although this signaling is known to downregulate cardiac Nav1.5, its regulation of Cx43 is unclear as conflicting results have been reported. The present study investigated how Wnt/β-catenin signaling regulates Cx43 in rat and human cardiomyocytes and if it is dependent on the sex of the cells or the metabolic substrates.
Methods
Male and female neonatal rat ventricular myocytes (NRVMs) were treated with CHIR-99021 (CHIR) or Wnt3a protein, two different activators of the Wnt/β-catenin signaling, either in a medium rich in glucose (a preferred metabolic substrate in heart failure) or in a medium rich in lipid (∼150 μM fatty acid, a preferred substrate in healthy hearts). Both healthy and Brugada Syndrome human iPSC-derived cardiomyocytes (iPSC-CMs) were used to confirm observations in NRVMs.
Results
When maintained in a glucose-rich medium, Gja1 mRNA (encoding Cx43) was reduced by a low concentration of CHIR (1 μM) in female NRVMs but only at a high concentration of CHIR (10 μM) in male NRVMs. However, reductions in Cx43 protein were observed at 1 μM CHIR in both male and female NRVMs, suggesting the involvement of both transcriptional and post-transcriptional mechanisms. When maintained in a lipid-rich medium, neither Gja1 mRNA nor Cx43 protein was altered by CHIR at 1 or 3 μM. In contrast, CHIR-induced reductions in Scn5a mRNA and Nav1.5 protein were observed in both glucose-rich and lipid-rich media, with no significant sex-specific differences detected. Consistent with studies using CHIR, which is a Wnt receptor-independent activator, Wnt3a protein also reduced both Gja1 mRNA and Cx43 protein in NRVMs in the glucose-rich medium but not in the lipid-rich medium. In human iPSC-CMs from two healthy volunteers and one Brugada Syndrome patient, Wnt/β-catenin signaling activation reduced GJA1 mRNA and Cx43 protein in a standard, glucose-containing medium.
Conclusions
These data demonstrate that metabolic substrates regulate the effects of Wnt/β-catenin signaling in cardiomyocytes, with reductions in Cx43 mRNA and protein only observed when glucose is the primary metabolic substrate, which occurs in arrhythmogenic conditions such as cardiac hypertrophy and heart failure.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of molecular and cellular cardiology plus
Cardiology and Cardiovascular Medicine
自引率
0.00%
发文量
0
审稿时长
31 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


