MIL-125催化合成1,2-环己二羧酸酯的酯化机理研究
IF 3
3区 化学
Q3 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
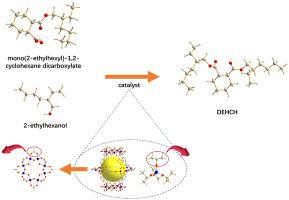
二(2-乙基己基)-1,2-环己烷二羧酸酯(DEHCH)有望作为一种无毒增塑剂取代邻苯二甲酸二(2-乙基己基)酯(DEHP)。在合成DEHCH的酯化过程中,二酯化过程通常被称为速率控制步骤。为了更好地理解这一过程,利用密度泛函理论(DFT)研究了钛酸四异丙基[Ti(OiPr)4]、MIL-125、MIL-125- nh2和水合MIL-125催化单(2-乙基己基)-1,2-环己烷二羧酸酯与2-乙基己醇的反应机理。结果表明,Ti(OiPr)4具有单一的配位不饱和Ti位点,反应遵循双分子亲核取代(SN2)机制,能垒高达184.97 kJ·mol−1。考虑到钛的高氧亲和性和金属有机骨架(mof)中反应位点的丰富性,我们创新性地选择了MIL-125作为研究对象。模拟结果表明,两个配位不饱和Ti原子之间的μ2-O桥接充当了一个额外的Brønsted碱基,提高了反应活性。同时,生成的μ2-OH辅助脱水,并以101.11 kJ·mol−1的能垒作为速率决定步骤。为了进一步改善脱水效果,有机配体上的-NH2官能团和产品水中解离的-OH官能团被策略性地用作不同的Brønsted碱基。在MIL-125-NH2中,脱水能垒降低到33.31 kJ·mol−1。值得注意的是,在水合MIL-125中,2-乙基己醇未被吸附,脱水能垒降至25.24 kJ·mol−1。这些结果表明MIL-125可以将产物水的不利影响转化为有利因素,因此MIL-125被推荐用于催化1,2-环己二羧酸与2-乙基己醇的酯化反应。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Study on the esterification mechanism catalyzed by MIL-125 for the synthesis of 1,2-cyclohexanedicarboxylates
Di(2-ethylhexyl)-1,2-cyclohexane dicarboxylate (DEHCH) is expected to function as a non-toxic plasticizer for replacing di(2-ethylhexyl) phthalate (DEHP). During the esterification for synthesis of DEHCH, the di-esterification process is usually known as rate control step. To better understand this process, Density Functional Theory (DFT) was utilized to investigate the mechanism of mono(2-ethylhexyl)-1,2-cyclohexane dicarboxylate with 2-ethylhexanol catalyzed by tetraisopropyl titanate [Ti(OiPr)4], MIL-125, MIL-125-NH2, and hydrated MIL-125. The results indicate that Ti(OiPr)4 possesses a single coordinatively unsaturated Ti site and the reaction follows the bimolecular nucleophilic substitution (SN2) mechanism, with an energy barrier as high as 184.97 kJ·mol−1. Given Ti's high oxygen affinity and the abundance of reaction sites in metal-organic frameworks (MOFs), MIL-125, was innovatively selected for investigation. Simulated results show that the μ2-O bridging between the two coordinatively unsaturated Ti atoms acts as an additional Brønsted base site, enhancing the reactivity. Meanwhile, the generated μ2-OH assists in dehydration, serving as the rate-determining step with an energy barrier of 101.11 kJ·mol−1. To further improve the dehydration, the -NH2 group functionalized on the organic ligand and the dissociated -OH group from product water were strategically utilized as distinct Brønsted base sites. In MIL-125-NH2, the energy barrier for dehydration was reduced to 33.31 kJ·mol−1. Notably, in hydrated MIL-125, 2-ethylhexanol remains unadsorbed, lowering the energy barrier for dehydration to 25.24 kJ·mol−1. These findings suggest that MIL-125 can transform the adverse impact of product water into a beneficial factor, thereby MIL-125 is recommended to catalyze the esterification of 1,2-cyclohexanedicarboxylic acid with 2-ethylhexanol.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Computational and Theoretical Chemistry
CHEMISTRY, PHYSICAL-
CiteScore
4.20
自引率
10.70%
发文量
331
审稿时长
31 days
期刊介绍:
Computational and Theoretical Chemistry publishes high quality, original reports of significance in computational and theoretical chemistry including those that deal with problems of structure, properties, energetics, weak interactions, reaction mechanisms, catalysis, and reaction rates involving atoms, molecules, clusters, surfaces, and bulk matter.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


