α-氨基-ε-己内酰胺在278.15 ~ 323.15 K 6种纯溶剂中溶解行为的实验与计算研究
IF 5.2
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
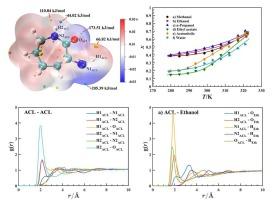
研究了α-氨基-ε-己内酰胺(ACL)在0.1 MPa、278.15 ~ 323.15 K条件下在甲醇、乙醇、正丙醇、乙酸乙酯、乙腈和水中的溶解行为。实验结果表明,ACL在所有溶剂中的溶解度均随温度升高而升高,在300 K以下的醇中溶解度明显升高,这是由于溶质-溶剂氢键相互作用更强。相反,在水中的低溶解度是由于水分子的强自结合。热力学模型相关性表明,Apelblat和Wilson模型提供了最佳拟合,而cosmos - rs预测再现了一般的温度和溶剂相关趋势,但精度较低。由van ' t Hoff方程得出的溶解性质表明,所有溶剂中的溶解过程都是吸热和熵驱动的,其中焓是主要的贡献者。这些结果为ACL在溶剂中的溶解提供了一致的理解,并为溶剂选择和结晶工艺设计提供了指导。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Experimental and computational study on the dissolution behavior of α-amino-ε-caprolactam in six pure solvents at 278.15 K to 323.15 K
The dissolution behavior of α-amino-ε-caprolactam (ACL) in methanol, ethanol, n-propanol, ethyl acetate, acetonitrile, and water was investigated over 278.15–323.15 K at 0.1 MPa using experimental and computational methods. Experimental results show that ACL solubility increases with temperature in all solvents and is significantly higher in alcohols below 300 K, owing to stronger solute–solvent hydrogen-bonding interactions. In contrast, the unexpectedly low solubility in water is attributed to strong self-association of water molecules. Thermodynamic model correlations demonstrated that the Apelblat and Wilson models provided the best fits, whereas the COSMO-RS predictions reproduced general temperature- and solvent-dependent trends but with lower accuracy. Dissolution properties derived from the van ’t Hoff equation indicated that the dissolution processes in all solvents were endothermic and entropy-driven, with enthalpy being the main contributor. These results provide a consistent understanding of ACL dissolution across solvents and offer guidance for solvent selection and crystallization process design.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Molecular Liquids
化学-物理:原子、分子和化学物理
CiteScore
10.30
自引率
16.70%
发文量
2597
审稿时长
78 days
期刊介绍:
The journal includes papers in the following areas:
– Simple organic liquids and mixtures
– Ionic liquids
– Surfactant solutions (including micelles and vesicles) and liquid interfaces
– Colloidal solutions and nanoparticles
– Thermotropic and lyotropic liquid crystals
– Ferrofluids
– Water, aqueous solutions and other hydrogen-bonded liquids
– Lubricants, polymer solutions and melts
– Molten metals and salts
– Phase transitions and critical phenomena in liquids and confined fluids
– Self assembly in complex liquids.– Biomolecules in solution
The emphasis is on the molecular (or microscopic) understanding of particular liquids or liquid systems, especially concerning structure, dynamics and intermolecular forces. The experimental techniques used may include:
– Conventional spectroscopy (mid-IR and far-IR, Raman, NMR, etc.)
– Non-linear optics and time resolved spectroscopy (psec, fsec, asec, ISRS, etc.)
– Light scattering (Rayleigh, Brillouin, PCS, etc.)
– Dielectric relaxation
– X-ray and neutron scattering and diffraction.
Experimental studies, computer simulations (MD or MC) and analytical theory will be considered for publication; papers just reporting experimental results that do not contribute to the understanding of the fundamentals of molecular and ionic liquids will not be accepted. Only papers of a non-routine nature and advancing the field will be considered for publication.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


