硫/氧置换合成Bi2S3/Bi2O3纳米催化剂的表征及其对环丙沙星光催化性能的响应面法优化
IF 5.2
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
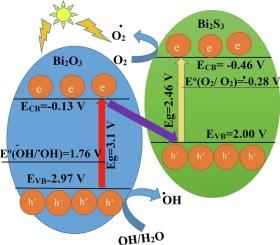
采用硫氧置换法制备了Bi2S3/Bi2O3纳米级偶联催化剂。采用扫描电镜(SEM)、x射线衍射(XRD)、紫外-可见漫反射光谱(DRS)和傅里叶变换红外光谱(FT-IR)对合成的Bi2O3和Bi2S3纳米粒子及其偶联催化剂进行了表征。采用Williamson-Hall (WH)和Scherrer模型测定Bi2S3、Bi2O3和Bi2S3/Bi2O3的晶粒尺寸分别为60、51和63 nm (WH模型)和50、42和37 nm (Scherrer模型)。DRS分析计算出Bi2S3和Bi2O3的能带能分别为2.46和3.1 eV。x射线图证实整个催化剂的组成元素分布相对均匀。Bi2S3、Bi2O3和Bi2S3/Bi2O3的pHpzc估计值分别为6.1、6.9和6.4。在最初的光降解实验中,获得了Bi2S3/Bi2O3对环丙沙星(CIP)的光催化活性增强。因此,通过响应面法(RSM)研究并优化了关键操作变量的同时效应。实验条件为:CIP溶液pH为10,浓度为12 mg/L,催化剂用量为1 g/L,光照时间为110 min。二阶多项式模型处理数据的相关系数较高,R2值为0.9994,证明了RSM预测数据与实验结果的一致性。过程动力学符合准一阶Hinshelwood模型。催化剂的稳定性得到了证实,因为在连续四个重复使用循环中,光降解活性没有显著差异。毕竟,在四次重复使用中,光降解活性没有显著差异。在清除率研究中,Na2SO4作为电子清除率的临界降低活性,证实了光诱导电子在二元催化剂光降解CIP中的相对最高作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Characterization of the sulfur/oxygen-displacement synthesized Bi2S3/Bi2O3 nano catalyst and response surface methodology optimization of its photocatalytic performance toward ciprofloxacin
The coupled Bi2S3/Bi2O3 nano-scale catalyst was fabricated via a sulfur/oxygen-displacement procedure. The as-synthesized Bi2O3 and Bi2S3 NPs and the coupled catalyst were identified by SEM, XRD, UV–Vis diffuse reflectance spectroscopy (DRS), and FT-IR. Crystallite size determination with Williamson-Hall (W![]() H) and Scherrer models gave values of 60, 51, and 63 nm (by W
H) and Scherrer models gave values of 60, 51, and 63 nm (by W![]() H model), and 50, 42, and 37 nm (by Scherrer model) for Bi2S3, Bi2O3, and Bi2S3/Bi2O3, respectively. Band gap energy of Bi2S3 and Bi2O3 calculated with DRS analysis is 2.46 and 3.1 eV. X-ray maps confirmed a relatively homogenous constituent elemental distribution throughout the catalyst. The pHpzc estimation for Bi2S3, Bi2O3, and Bi2S3/Bi2O3 gave values of 6.1, 6.9, and 6.4, respectively. In the initial photodegradation experiments, the boosted photocatalytic activity of Bi2S3/Bi2O3 was obtained toward ciprofloxacin (CIP). Thus, the simultaneous effects of the vital operating variables were investigated and optimized via a response surface methodology (RSM) approach. The RSM optimal run was defined at the experimental conditions of 12 mg/L CIP solution at pH 10, catalyst dose of 1 g/L, and illumination time of 110 min. The satisfactory correlation coefficient for the applicability of the second-order polynomial model to process the data was confirmed by a high R2 value of 0.9994, which proves the agreement between the RSM predicted data and the experimental results. The process kinetics was well fitted to the pseudo-first-order Hinshelwood model. Catalyst stability was confirmed because no significant difference was observed in the photo degradation activity over four consecutive reusing cycles. After all, no significant difference was observed in the photodegradation activity over four reusing runs. In the scavenging agents study, the critical decreased activity by Na2SO4, as an electron scavenging agent, confirmed the highest relative role of the photoinduced electrons in CIP photodegradation by the binary catalyst used.
H model), and 50, 42, and 37 nm (by Scherrer model) for Bi2S3, Bi2O3, and Bi2S3/Bi2O3, respectively. Band gap energy of Bi2S3 and Bi2O3 calculated with DRS analysis is 2.46 and 3.1 eV. X-ray maps confirmed a relatively homogenous constituent elemental distribution throughout the catalyst. The pHpzc estimation for Bi2S3, Bi2O3, and Bi2S3/Bi2O3 gave values of 6.1, 6.9, and 6.4, respectively. In the initial photodegradation experiments, the boosted photocatalytic activity of Bi2S3/Bi2O3 was obtained toward ciprofloxacin (CIP). Thus, the simultaneous effects of the vital operating variables were investigated and optimized via a response surface methodology (RSM) approach. The RSM optimal run was defined at the experimental conditions of 12 mg/L CIP solution at pH 10, catalyst dose of 1 g/L, and illumination time of 110 min. The satisfactory correlation coefficient for the applicability of the second-order polynomial model to process the data was confirmed by a high R2 value of 0.9994, which proves the agreement between the RSM predicted data and the experimental results. The process kinetics was well fitted to the pseudo-first-order Hinshelwood model. Catalyst stability was confirmed because no significant difference was observed in the photo degradation activity over four consecutive reusing cycles. After all, no significant difference was observed in the photodegradation activity over four reusing runs. In the scavenging agents study, the critical decreased activity by Na2SO4, as an electron scavenging agent, confirmed the highest relative role of the photoinduced electrons in CIP photodegradation by the binary catalyst used.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Molecular Liquids
化学-物理:原子、分子和化学物理
CiteScore
10.30
自引率
16.70%
发文量
2597
审稿时长
78 days
期刊介绍:
The journal includes papers in the following areas:
– Simple organic liquids and mixtures
– Ionic liquids
– Surfactant solutions (including micelles and vesicles) and liquid interfaces
– Colloidal solutions and nanoparticles
– Thermotropic and lyotropic liquid crystals
– Ferrofluids
– Water, aqueous solutions and other hydrogen-bonded liquids
– Lubricants, polymer solutions and melts
– Molten metals and salts
– Phase transitions and critical phenomena in liquids and confined fluids
– Self assembly in complex liquids.– Biomolecules in solution
The emphasis is on the molecular (or microscopic) understanding of particular liquids or liquid systems, especially concerning structure, dynamics and intermolecular forces. The experimental techniques used may include:
– Conventional spectroscopy (mid-IR and far-IR, Raman, NMR, etc.)
– Non-linear optics and time resolved spectroscopy (psec, fsec, asec, ISRS, etc.)
– Light scattering (Rayleigh, Brillouin, PCS, etc.)
– Dielectric relaxation
– X-ray and neutron scattering and diffraction.
Experimental studies, computer simulations (MD or MC) and analytical theory will be considered for publication; papers just reporting experimental results that do not contribute to the understanding of the fundamentals of molecular and ionic liquids will not be accepted. Only papers of a non-routine nature and advancing the field will be considered for publication.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


