lorundrostat在不受控制或顽固性高血压患者中的疗效和安全性:一项系统评价和荟萃分析与试验序贯分析。
IF 3.5
3区 医学
Q2 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
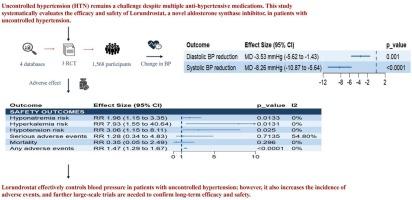
背景:尽管有多种抗高血压药物,但不受控制的高血压(HTN)仍然是一个挑战。本研究系统评价了Lorundrostat(一种新型醛固酮合成酶抑制剂)在未控制的高血压患者中的疗效和安全性。方法:到2025年7月14日,对主要电子数据库进行全面检索,以确定Lorundrostat与安慰剂的随机对照试验(rct)。主要结局包括办公室收缩压和舒张压(BP)的变化。随机效应模型用于汇总数据,以95% %置信区间(ci)的风险比(RR)或平均差异(MD)表示。结果:共纳入1562例患者的3项随机对照试验的汇总分析显示,lorundrostat可显著降低两组患者的有效收缩压(MD = -8.26 mmHg; 95% % CI: -10.87 ~ -5.64; p )结论:lorundrostat可有效控制高血压患者的血压,但也增加了不良事件的发生率,需要进一步的大规模试验来证实其长期疗效和安全性。普洛斯彼罗id: CRD420251107424。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Efficacy and safety of lorundrostat in patients with uncontrolled or resistant hypertension: A systematic review and meta-analysis with trial sequential analysis
Background
Uncontrolled hypertension (HTN) remains a challenge despite multiple anti-hypertensive medications. This study systematically evaluates the efficacy and safety of Lorundrostat, a novel aldosterone synthase inhibitor, in patients with uncontrolled hypertension.
Methods
A comprehensive search of major electronic databases was conducted until Jul 14, 2025, to identify randomized controlled trials (RCTs) comparing Lorundrostat with placebo. The primary outcomes included changes in office systolic and diastolic blood pressure (BP). A random-effects model was used to pool the data, presented as risk ratios (RR) or mean differences (MD) with 95 % confidence intervals (CIs).
Results
The pooled analysis of three RCTs comprising 1562 patients demonstrated that lorundrostat yielded statistically significant reductions in both office systolic BP (MD = −8.26 mmHg; 95 % CI: −10.87 to −5.64; p < 0.0001) and diastolic BP (MD = −3.53 mmHg; 95 % CI: −5.62 to −1.43; p = 0.001). However, Lorundrostat was associated with increased risks of hyperkalemia (RR = 7.93; 95 % CI: 1.55 to 40.64; p = 0.0131), hyponatremia (RR = 1.96; 95 % CI: 1.15 to 3.35; p = 0.0133), hypotension (RR = 3.06; 95 % CI: 1.15 to 8.11; p = 0.0250), and any adverse events (RR = 1.47; 95 % CI: 1.29 to 1.67; p < 0.0001).
Conclusion
Lorundrostat effectively controls blood pressure in patients with uncontrolled hypertension; however, it also increases the incidence of adverse events, and further large-scale trials are needed to confirm long-term efficacy and safety.
PROSPERO ID: CRD420251107424.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Vascular pharmacology
医学-药学
CiteScore
6.60
自引率
2.50%
发文量
153
审稿时长
31 days
期刊介绍:
Vascular Pharmacology publishes papers, which contains results of all aspects of biology and pharmacology of the vascular system.
Papers are encouraged in basic, translational and clinical aspects of Vascular Biology and Pharmacology, utilizing approaches ranging from molecular biology to integrative physiology. All papers are in English.
The Journal publishes review articles which include vascular aspects of thrombosis, inflammation, cell signalling, atherosclerosis, and lipid metabolism.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


