肝乌头酶1重定向柠檬酸通量抑制脂肪生成和改善高胆固醇血症。
IF 11.9
1区 医学
Q1 ENDOCRINOLOGY & METABOLISM
引用次数: 0
摘要
背景与目的:靶向肝新生脂肪生成(DNL)中的关键酶是治疗高胆固醇血症的一种有前景的策略。然而,控制肝脏DNL的精确调控机制仍不完全清楚。胞质柠檬酸盐在DNL中起着至关重要的作用,其中柠檬酸盐代谢的关键酶aconitase 1 (ACO1)可能影响脂质代谢。本研究的目的是阐明肝脏ACO1在调节肝脏和全身脂质稳态中的作用。方法:测定多种高胆固醇血症动物模型肝组织中ACO1的表达和活性。通过肝脏特异性基因操作,我们研究了肝脏ACO1基因敲除和过表达对高胆固醇血症和动脉粥样硬化的影响。靶向代谢组学和基于稳定同位素的通量分析用于分析肝脏底物利用模式。结果:在高胆固醇血症患者和动物模型中,肝脏ACO1表达均显著降低。在小鼠模型中,肝细胞特异性ACO1缺失加重了血脂异常,而ACO1过表达改善了高胆固醇血症、肝脂肪变性和动脉粥样硬化。从机制上讲,ACO1过表达将胞质柠檬酸盐代谢转向α-酮戊二酸,从而限制了乙酰辅酶a对DNL的可用性,抑制了脂肪酸和胆固醇的合成。这些降脂效果依赖于ACO1酶活性,因为催化活性不强的ACO1突变体无法复制所观察到的益处。结论:我们的研究结果表明,肝脏ACO1是脂质代谢稳态的关键调节因子。促进ACO1介导的柠檬酸盐重定向通过抑制肝脏DNL有效减轻高胆固醇血症和动脉粥样硬化,突出表明ACO1是降脂治疗的潜在靶点。本文章由计算机程序翻译,如有差异,请以英文原文为准。
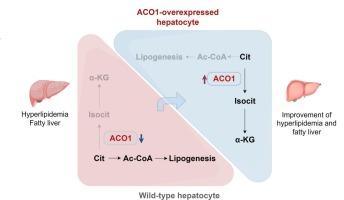
Hepatic aconitase 1 redirects citrate flux to suppress lipogenesis and ameliorate hypercholesterolemia
Background and aims
Targeting key enzymes in hepatic de novo lipogenesis (DNL) presents a promising strategy for treating hypercholesterolemia. However, the precise regulatory mechanisms governing hepatic DNL remain incompletely understood. Cytosolic citrate plays a crucial role in DNL, with aconitase 1 (ACO1), a key enzyme in citrate metabolism, potentially influencing lipid metabolism. The aim of this study was to clarify the role of hepatic ACO1 in regulating both hepatic and systemic lipid homeostasis.
Methods
ACO1 expression and activity were assessed in liver tissues from multiple hypercholesterolemic animal models. Using liver-specific genetic manipulation, we examined the effects of hepatic ACO1 knockout and overexpression on hypercholesterolemia and atherosclerosis. Targeted metabolomics and stable isotope-based flux analysis were used to profile hepatic substrate utilization patterns.
Results
Hepatic ACO1 expression was significantly reduced in both hypercholesterolemic patients and animal models. Hepatocyte-specific ACO1 deletion exacerbated dyslipidemia, while ACO1 overexpression improved hypercholesterolemia, hepatic steatosis, and atherosclerosis in mouse models. Mechanistically, ACO1 overexpression redirected cytosolic citrate metabolism toward α-ketoglutarate, thereby limiting acetyl-CoA availability for DNL and suppressing fatty acid and cholesterol synthesis. These lipid-lowering effects were dependent on ACO1 enzymatic activity, as catalytically inactive ACO1 mutants failed to replicate the observed benefits.
Conclusion
Our findings identify hepatic ACO1 as a critical regulator of lipid metabolism homeostasis. Promoting ACO1-mediated citrate redirection effectively mitigates hypercholesterolemia and atherosclerosis by suppressing hepatic DNL, highlighting ACO1 as a potential target for lipid-lowering therapies.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Metabolism: clinical and experimental
医学-内分泌学与代谢
CiteScore
18.90
自引率
3.10%
发文量
310
审稿时长
16 days
期刊介绍:
Metabolism upholds research excellence by disseminating high-quality original research, reviews, editorials, and commentaries covering all facets of human metabolism.
Consideration for publication in Metabolism extends to studies in humans, animal, and cellular models, with a particular emphasis on work demonstrating strong translational potential.
The journal addresses a range of topics, including:
- Energy Expenditure and Obesity
- Metabolic Syndrome, Prediabetes, and Diabetes
- Nutrition, Exercise, and the Environment
- Genetics and Genomics, Proteomics, and Metabolomics
- Carbohydrate, Lipid, and Protein Metabolism
- Endocrinology and Hypertension
- Mineral and Bone Metabolism
- Cardiovascular Diseases and Malignancies
- Inflammation in metabolism and immunometabolism
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


