原子精密铜簇用于高化学选择性的类碳化合物插入脂肪胺的N-H键
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
由于脂肪胺与过渡金属催化剂之间具有较强的配位作用,因此脂肪胺的强路易斯碱度抑制了催化剂的活性,导致催化剂的化学选择性较差,对N-H插入反应构成了较大的挑战。在这里,我们首次报道了一个具有动态催化位点的高稳定铜簇(Cu3NC(NHC)),它被用于高效和化学选择性地催化脂肪胺的N-H插入反应。吡啶基团作为动态配体和脂肪胺可以竞争配位,以防止催化剂钝化失活。解离的动态配体可以调节催化剂的空间效应,实现伯胺的高选择性烷基化。此外,该反应通过形成Cu3NC(NHC)-类碳中间体,在C = C键和N-H键之间具有较高的化学选择性。N-H插入反应的TON达到79200。该反应可用于药物合成和后期功能化。机理研究和密度泛函理论表明,这一生成Cu3NC(NHC)-类碳化合物的过程在动力学和热力学上都是有利的,这一过程可能不是限速步骤。该研究突出了金属纳米团簇在有机催化领域的广阔应用前景,为团簇化学的发展提供了新的思路。本文章由计算机程序翻译,如有差异,请以英文原文为准。
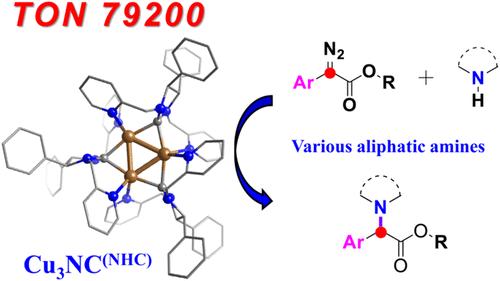
Atomically Precise Copper Cluster for Highly Chemoselective Carbenoid Insertion into the N–H Bond of Aliphatic Amine
Due to the strong coordination effect between aliphatic amines and transition metal catalysts, the activity of the catalyst is inhibited by aliphatic amines’ strong Lewis basicity, leading to poor chemoselectivity and a significant challenge to the N–H insertion reaction. Here, we report a highly stable copper cluster (Cu3NC(NHC)) with dynamic catalytic sites for the first time that is used to efficiently and chemoselectively catalyze the N–H insertion reaction of aliphatic amines. Pyridine groups as dynamic ligands and aliphatic amines could compete for coordination to prevent catalyst deactivation due to passivation. The dissociated dynamic ligands could regulate the steric effect of the catalyst to achieve highly selective alkylation of primary amines. Moreover, the reaction afforded high chemoselectivity between the C═C bond and the N–H bond by forming a Cu3NC(NHC)-carbenoid intermediate. TON of the N–H insertion reaction has reached 79200. The reaction could be applied in pharmaceutical synthesis and late-stage functionalization. Mechanistic studies and density functional theory demonstrate that this process of generating Cu3NC(NHC)-carbenoid was both kinetically and thermodynamically favorable and that this process may not be the rate-limiting step. This investigation highlights the broad application prospects of metal nanoclusters in the field of organic catalysis, providing a new idea for the development of cluster chemistry.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


