一步合成氰化喹啉区域异构体使n型聚合物具有创纪录的环境处理热电性能
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
有机电子学的进步主要依赖于高性能n型π共轭聚合物的发展。然而,由于获得满足这些要求的构建块的限制,这种具有易于合成,优异的可加工性和良好的空气稳定性的电子耗尽聚合物仍然很少见。在这里,我们开发了两种新颖的,结构简单的,但π扩展和高度缺电子的氰化构建块:2,3-喹啉二碳腈(QxCN)和6,7-喹啉二碳腈(CNQx)。这些单位是通过弱受体喹啉的区域选择性氰化,从低成本前体一步合成的。与噻吩双酮吡咯共聚得到两种受体-受体型共聚物TDPP-QxCN和TDPP-CNQx,与非氰化聚合物类似物TDPP-Qx (- 3.35 eV)相比,LUMO水平明显更高(~ - 3.9 eV)。深入的光谱、理论和微观结构分析表明,在TDPP-CNQx的6,7位氰化抑制了TDPP-QxCN中与分子内电荷转移相关的少数载流子效应,同时促进了出色的构象适应性,从而驱动高效的自组装成具有良好平衡的有序和无序结构域的高度结晶膜。这种微观结构组织确保了快速的电子传递和有效的掺杂扩散,从而导致TDPP-CNQx的大场效应迁移率(高达1.3 cm2 V-1 s-1)和前所未有的n掺杂效率,即使在空气处理条件下也是如此。因此,n掺杂的TDPP-CNQx薄膜表现出高n型电导率(44.50 S cm-1),优异的功率因数(65.16 μW m-1 K-2)和超低的导热系数(0.10 W m-1 K-1),最终在溶液/环境处理薄膜中达到创纪录的热电性能值(ZT = 0.20)。这种性能也超过了几乎所有真空处理的同行,将CNQx定位为下一代有机电子产品的极有前途的构建块。本文章由计算机程序翻译,如有差异,请以英文原文为准。
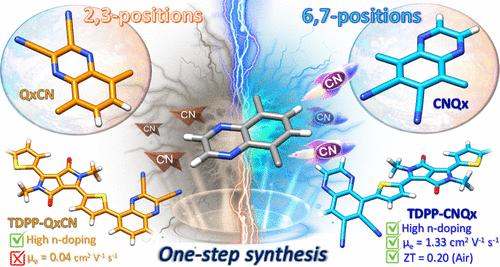
One-Step Synthesis of Cyanated Quinoxaline Regioisomers Enables n-Type Polymers with Record Ambient-Processed Thermoelectric Performance
Advancements in organic electronics critically depend on the development of high-performance n-type π-conjugated polymers. However, electron-depleted polymers of this kind exhibiting facile synthesis, excellent processability and good air stability remain rare due to limitations in accessing building blocks meeting these requirements. Here we develop two novel, structurally simple and yet π-extended and highly electron-deficient cyanated building blocks: 2,3-quinoxalinedicarbonitrile (QxCN) and 6,7-quinoxalinedicarbonitrile (CNQx). These units are synthesized in a single-step from low-cost precursors via regioselective cyanation of the weak acceptor quinoxaline. Copolymerization with thiophene-flanked diketopyrrolopyrrole affords two acceptor–acceptor type copolymers TDPP-QxCN and TDPP-CNQx, respectively, exhibiting significantly deeper LUMO levels (∼−3.9 eV) compared to the noncyanated polymer analogue TDPP-Qx (−3.35 eV). In-depth spectroscopic, theoretical, and microstructural analyses reveal that cyanation at the 6,7-positions in TDPP-CNQx suppresses minority carrier effects associated with intramolecular charge transfer in TDPP-QxCN, while promoting excellent conformational adaptability that drives efficient self-assembly into a highly crystalline film with a favorable balance of ordered and disordered domains. This microstructural organization ensures rapid electron transport and efficient dopant diffusion, leading to large field-effect mobilitiesfor TDPP-CNQx (up to 1.3 cm2 V–1 s–1) and unprecedented n-doping efficiency even under air-processed conditions. Consequently, n-doped TDPP-CNQx films exhibit a high n-type electrical conductivity (44.50 S cm–1), excellent power factor (65.16 μW m–1 K–2), and ultralow thermal conductivity (0.10 W m–1 K–1), collectively culminating in a record thermoelectric figure of merit (ZT = 0.20) for solution/ambient-processed films. This performance also surpasses nearly all vacuum-processed counterparts, positioning CNQx as a highly promising building block for next-generation organic electronics.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


