利用工程亚胺还原酶和氧化还原缓冲实现C(sp3) -H键的光生物催化自由基氢烷基化
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
利用烟酰胺或黄素依赖的氧化还原酶的光生物催化系统使烯烃的各种加氢功能化成为可能。然而,这些通常依赖于预激活的自由基前体和天然还原等量物(例如,还原性烟酰胺腺嘌呤二核苷酸磷酸(NADPH))。利用丰富的C-H底物进行直接氢烷基化仍然具有吸引力,但也极具挑战性。在这里,我们报道了一种光生物催化“氧化还原缓冲”策略,该策略重新利用亚胺还原酶(IRED)来催化与商业烷烃的酰胺的氢烷基化。协同外部过氧化物和合成光催化剂通过氢原子转移(HAT)激活C(sp3) -H键,而工程IRED与其天然NADPH精确协调随后的C自由基加入到酰胺和立体决定HAT步骤。这种外部氧化剂与天然还原剂的协同整合,再加上光氧化还原和生物催化的合并,解锁了非天然的酶功能,产生了对映体富集胺(ee高达99%,IRED低至0.02 mol %)。本文章由计算机程序翻译,如有差异,请以英文原文为准。
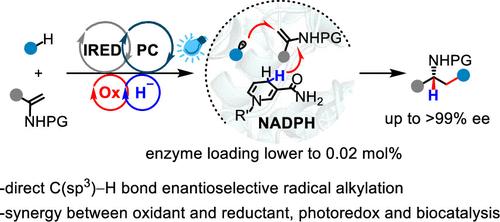
Photobiocatalytic Radical Hydroalkylation with C(sp3)–H Bonds Enabled by Engineered Imine Reductase and Redox Buffering
Photobiocatalytic systems that leverage nicotinamide- or flavin-dependent oxidoreductases have enabled diverse hydro-functionalization of alkenes. However, these typically rely on preactivated radical precursors and native reducing equivalents (e.g., reduced nicotinamide adenine dinucleotide phosphate (NADPH)). Direct hydroalkylation using abundant C–H substrates remains attractive yet highly challenging. Here, we report a photobiocatalytic “redox buffering” strategy that repurposes imine reductase (IRED) to catalyze the hydroalkylation of enamides with commercial alkanes. Cooperative external peroxide and synthetic photocatalyst activate C(sp3)–H bonds via hydrogen atom transfer (HAT), while engineered IRED with its native NADPH precisely orchestrates subsequent C-radical addition to enamides and stereodetermining HAT steps. This synergistic integration of an external oxidant with the native reductant, coupled with the merging of photoredox and biocatalysis, unlocks unnatural enzyme functionalities, producing enantioenriched amines (with up to >99% ee, low to 0.02 mol % of IRED).
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


