脂质囊泡收缩和粘附的动态形状调节
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
脂质膜结合的细胞器通常具有复杂的形态,具有空间变化的曲率和相对于内部体积(小体积)的大膜表面积。这些特征被认为是蛋白质分选和囊泡运输所必需的,但在体外繁殖具有挑战性。在这里,我们发现弱粘附的巨大单层囊泡(guv)可以被渗透收缩到低至0.1的体积,类似于扁平的圆盘状细胞器,如高尔基池和ER片。通过Canham-Helfrich模型的形状分析,我们确定了包括粘附强度、膜张力和单个囊泡压力在内的力学参数。我们发现,在放气过程中,形状扁平化的速度是由一个标准化的附着强度决定的,它结合了囊泡大小、附着能和弯曲刚度。对于高度扁平的圆盘状囊泡,我们确定了一种几何关系,该关系允许仅从囊泡的纵横比、大小和弯曲刚度来估计粘附强度。这些结果为自下而上研究膜形成机制和形状依赖现象(如曲率介导的蛋白质分选)提供了定量实验平台。本文章由计算机程序翻译,如有差异,请以英文原文为准。
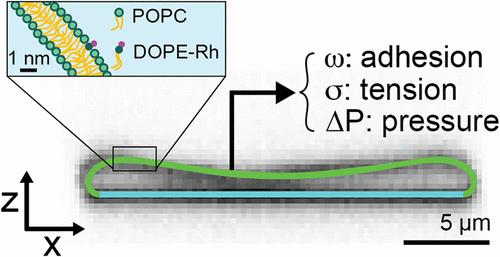
Dynamic Shape Modulation of Deflated and Adhered Lipid Vesicles
Lipid membrane-bounded organelles often possess intricate morphologies with spatially varying curvatures and large membrane surface areas relative to internal volume (small reduced volumes). These features are thought to be essential for protein sorting and vesicle trafficking, but challenging to reproduce in vitro. Here, we show that weakly adhered giant unilamellar vesicles (GUVs) can be osmotically deflated to reduced volumes as low as 0.1, similar to what is found in flattened, disc-shaped organelles such as Golgi cisternae and ER sheets. Using shape analysis with the Canham-Helfrich model, we determine mechanical parameters including adhesion strength, membrane tension, and pressure of individual vesicles. We find that the rate of shape flattening during deflation is governed by a normalized adhesion strength that combines vesicle size, adhesion energy, and bending rigidity. For highly flattened disc-like vesicles, we identify a geometric relationship that allows the adhesion strength to be estimated solely from the vesicle’s aspect ratio, size, and bending rigidity. These results provide a quantitative experimental platform for bottom-up studies of membrane shaping mechanisms and shape-dependent phenomena, such as curvature-mediated protein sorting.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


