氢化钯纳米粒子中的氢原子超晶格和三维不均匀性
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
当氢原子占据金属晶格的间隙位置时,它们形成金属氢化物(MHx),其结构和电子性质与宿主金属有很大不同。确定氢在MHx中的位置对于预测和理解氢化物的独特物理和电子性质至关重要。然而,由于传统的x射线和电子成像技术信号微弱,直接对宿主材料中的氢进行成像仍然是一个重大挑战。在这里,我们采用扫描透射电子显微镜(STEM)技术,对氢化钯(PdHx)纳米立方中H原子的三维(3D)分布进行了成像,PdHx是研究最多和工业相关的MHx材料之一。我们观察到氢在PdHx纳米立方体内的一维超晶格有序,以及PdHx纳米立方体内局部区域的三维氢聚集,揭示了金属氢化物纳米颗粒的空间异质性,这是以前通过其他方法无法获得的。本文章由计算机程序翻译,如有差异,请以英文原文为准。
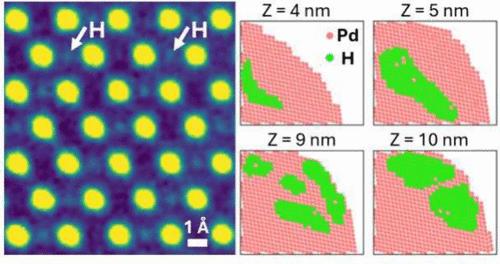
Electron Ptychography Images Hydrogen Atom Superlattices and 3D Inhomogeneities in Palladium Hydride Nanoparticles
When hydrogen atoms occupy interstitial sites in metal lattices, they form metal hydrides (MHx), whose structural and electronic properties can differ significantly from those of the host metals. Determining where the hydrogen is located within the MHx is crucial for predicting and understanding the resultant unique physical and electronic properties of the hydride. Yet, directly imaging hydrogen within a host material remains a major challenge due to its weak signal in conventional X-ray and electron imaging techniques. Here, we employ electron ptychography, a scanning transmission electron microscopy (STEM) technique, to image the three-dimensional (3D) distribution of H atoms in palladium hydride (PdHx) nanocubes, one of the most studied and industrially relevant MHx materials. We observe an unexpected one-dimensional superlattice ordering of hydrogen within the PdHx nanocubes and 3D hydrogen clustering in localized regions within the PdHx nanocubes, revealing spatial heterogeneity in metal hydride nanoparticles previously inaccessible by other methods.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


