细胞银嘧啶的化学机制途径
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
Argpyrimidine (APY)是一种甲基乙二醛衍生的晚期糖基化终产物(AGE),与多种疾病相关。由于APY是在没有酶的情况下形成的,因此无论是在单个蛋白质上还是在细胞中,都很难确定APY可能存在的位置。在这项研究中,我们使用肽模型系统和质谱分析来研究APY由甲基乙二醛(MGO)产生的化学机制,甲基乙二醛是一种生物相关的糖基化剂。与其他提出的APY形成机制一致,我们的研究结果确定了质量变化为[M + 144]的AGE物种,可能包括四氢嘧啶(THP),作为APY的直接前体。然而,我们的结果排除了先前提出的还原或氧化脱羧机制。相反,我们表明不需要正式的氧化步骤,并且释放甲酸而不是二氧化碳。我们进一步展示了附近残基(如Tyr)作为一般碱基协助APY形成机制的潜力。这些实验还表明,磷酸化的Tyr或Ser残基也可以促进等量的APY形成,尽管引入了我们之前发现的阻碍糖基化的额外负电荷。在这些机制见解和糖基化底物磷酸化残基新定义的作用的指导下,我们对mgo处理的细胞进行了定量的自下而上的蛋白质组学分析。对apy修饰蛋白的基因本体和功能注释聚类分析表明,与磷酸化相关的术语(例如,激酶活性或蛋白质磷酸化)存在相关性,这一点通过合成的磷酸肽底物得到了验证。总的来说,这些数据定义了APY的化学机制路径,并表明细胞磷酸化和糖基化事件(包括APY的形成)之间存在显著的串扰。本文章由计算机程序翻译,如有差异,请以英文原文为准。
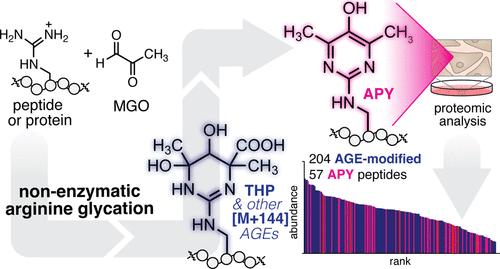
A Chemical Mechanistic Path Leads the Way to Cellular Argpyrimidine
Argpyrimidine (APY) is a methylglyoxal-derived advanced glycation end-product (AGE) that has been associated with multiple diseases. As APY forms without an enzyme, it remains exceptionally difficult to pinpoint where APY is likely to be found, both on individual proteins and in cells. In this study, we used a peptide model system and mass spectrometry analysis to investigate the chemical mechanism through which APY arises from methylglyoxal (MGO), a biologically relevant glycating agent. Consistent with other proposed APY formation mechanisms, our results identify AGE species with a mass change of [M + 144], presumably including tetrahydropyrimidine (THP), as a direct precursor to APY. However, our results rule out previously proposed reductone or oxidative decarboxylation mechanisms. Instead, we show that a formal oxidation step is not required, and that formate is released instead of CO2. We further show the potential for a nearby residue such as Tyr to assist in the APY formation mechanism by acting as a general base. These experiments also reveal that phosphorylated Tyr or Ser residues can also promote equivalent levels of APY formation, despite introducing additional negative charges that we previously showed to impede glycation. Guided by these mechanistic insights and a newly defined role for phosphorylated residues on glycation substrates, we performed quantitative bottom-up proteomics analysis for MGO-treated cells. Gene ontology and functional annotation clustering analyses for APY-modified proteins suggested a correlation with phosphorylation-related terms (e.g., kinase activity or protein phosphorylation), which was validated using synthetic phosphopeptide substrates. Collectively, these data define a chemical mechanistic path to APY and suggest significant crosstalk between cellular phosphorylation and glycation events including APY formation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


