li20 - b2o3 - lif体系中的LBO结晶
IF 1.4
4区 化学
Q4 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
研究了Li2O-B2O3-LiF三元体系的相平衡。结果表明,LBO初晶区具有较窄的浓度区间,受Li3B7O12-LiF线和Li2B8O18-LiF线的限制。研究了liff在空气中的加氢热解。实验表明,LiBO2-LiF熔体在800℃时LiF的分解不明显,但在900℃时LiF含量随时间的增加而显著降低。本文章由计算机程序翻译,如有差异,请以英文原文为准。
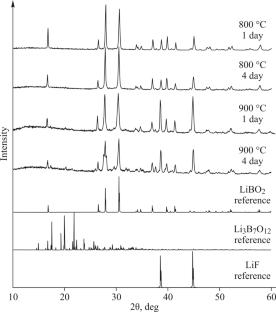
LBO Crystallization in the Li2O–B2O3–LiF System
Phase equilibria in the Li2O–B2O3–LiF ternary system are studied. It is established that the LBO primary crystallization region has a narrow concentration interval limited by Li3B7O12–LiF and Li2B8O18–LiF lines. The hydropyrolysis of LiF in air is studied. It is shown experimentally that the decomposition of LiF from the LiBO2–LiF melt at 800 °C is insignificant, but the LiF content decreases notably with time at 900 °C.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Structural Chemistry
化学-无机化学与核化学
CiteScore
1.60
自引率
12.50%
发文量
142
审稿时长
8.3 months
期刊介绍:
Journal is an interdisciplinary publication covering all aspects of structural chemistry, including the theory of molecular structure and chemical bond; the use of physical methods to study the electronic and spatial structure of chemical species; structural features of liquids, solutions, surfaces, supramolecular systems, nano- and solid materials; and the crystal structure of solids.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


