无保护醛糖合成1,2-顺式c -乙烯基呋喃苷的仿生工艺
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
c -糖苷是O-糖苷和n -糖苷的有价值的类似物,具有广泛的药物相关性。虽然大多数c -糖苷的合成方法依赖于受保护的糖基供体,但人们对利用未受保护的天然糖的方法越来越感兴趣,特别是用于合成c -呋喃苷的方法,因为c -呋喃苷在热力学上不如它们的吡喃基对应物更受欢迎。受酶促生物合成的启发,我们报道了一种仿生的“开闭”策略,用于从无保护的醛糖合成1,2-顺式c -乙烯基呋喃苷。该过程涉及醛糖和乙烯基硼酸之间的morpholine介导的Petasis反应,将环半缩醛转化为具有高1,2-抗选择性的线性多羟基胺。随后的二氟化苯诱导脱氨环有效地形成具有高化学选择性和非对映选择性的呋喃糖环。该方法具有广泛的底物范围,可容纳各种乙烯基硼酸以及天然单糖和双糖,并且可在温和的水相容条件下放大。乙烯基的下游衍生化提供1,2-顺式c -烷基呋喃苷,提供了有效的途径,否则具有挑战性的分子结构。本文章由计算机程序翻译,如有差异,请以英文原文为准。
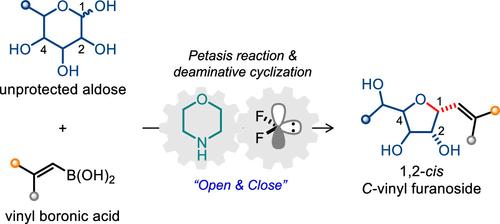
A Biomimetic Procedure for the Synthesis of 1,2-cis C-Vinyl Furanosides from Unprotected Aldoses
C-glycosides are valuable analogs of O- and N-glycosides with broad pharmaceutical relevance. While most synthetic approaches to C-glycosides rely on protected glycosyl donors, there is growing interest in methods that utilize unprotected native sugars, particularly for synthesizing C-furanosides, which are thermodynamically less favored than their pyranosyl counterparts. Inspired by enzymatic biosynthesis, we report a biomimetic “open-and-close” strategy for the synthesis of 1,2-cis C-vinyl furanosides from unprotected aldoses. This process involves a morpholine-mediated Petasis reaction between aldoses and vinyl boronic acids, converting cyclic hemiacetals into linear polyhydroxy amines with high 1,2-anti selectivity. Subsequent difluorocarbene-induced deaminative cyclization efficiently forms the furanose ring with high chemoselectivity and diastereoselectivity. The method exhibits broad substrate scope, accommodating various vinyl boronic acids as well as native mono- and disaccharides, and is amenable to scale-up under mild, aqueous-compatible conditions. Downstream derivatizations of the vinyl group afford 1,2-cis C-alkyl furanosides, offering efficient access to otherwise challenging molecular architectures.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


