集成溶剂化化学实现高能锂金属电池
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
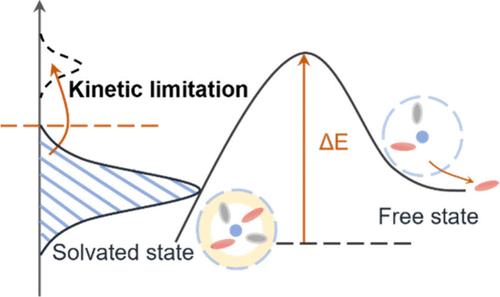
合理的高能锂金属电池电解液设计要求同时抑制溶剂反应性和促进阴离子还原。然而,现有的方法往往由于对溶剂化结构控制不足,导致游离溶剂和阴离子种类过多,副反应不断。在这里,我们提出了一种溶剂化整合策略,将溶剂分子和阴离子都限制在第一溶剂化鞘中,从而最大限度地减少它们的反应性。作为概念证明,我们使用亲锂(三氟甲氧基)氟苯(TFMFB)通过增强溶剂对Li+的亲和力来调节Li+溶剂化,确保有利的Li+动力学。同时,亲脂性(三氟甲基)环己烷(FMCH)形成一个外层动力学屏障,抑制溶剂/阴离子脱溶。这种内部亲石和外部亲脂的结构产生了协同约束,显著提高了氧化稳定性,实现了强大的界面化学。结果表明,使用定制溶剂集成电解质(SIE)的无阳极Cu∥LiNi0.5Co0.2Mn0.3O2 (NCM523)袋状电池在120次循环后容量保持率达到80%。此外,采用超低电解质(1.1 g Ah - 1)的10 Ah Li∥LiNi0.9Co0.05Mn0.05O2 (NCM955)袋状电池可提供568 Wh kg-1的超高能量密度。这种溶剂化集成的概念为实际高能lmb的电解质设计提供了有希望的指导。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Integrated Solvation Chemistry Enables High Energy Li Metal Batteries
Rational electrolyte design for high-energy Li metal batteries (LMBs) demands the simultaneous suppression of solvent reactivity and the promotion of anion reduction. However, existing approaches often fall short due to inadequate control over the solvation structure, leading to excessive free solvent and anion species and continual side reactions. Here, we propose a solvation integration strategy that confines both solvent molecules and anions within the first solvation sheath, thereby minimizing their reactivity. As a proof of concept, we employ lithiophilic (trifluoromethoxy)fluorobenzene (TFMFB) to tune Li+ solvation by enhancing the affinity of solvents toward Li+, ensuring favorable Li+ kinetics. Simultaneously, lipophilic (trifluoromethyl)cyclohexane (FMCH) forms an outer-layer kinetic barrier that inhibits solvent/anion desolvation. This inner-lithiophilic and outer-lipophilic configuration creates synergistic confinement, significantly enhancing oxidation stability and enabling robust interfacial chemistry. As a result, an anode-free Cu∥LiNi0.5Co0.2Mn0.3O2 (NCM523) pouch cell utilizing the tailored solvation-integrated electrolyte (SIE) achieves 80% capacity retention after 120 cycles. Furthermore, the aggressive 10 Ah Li∥LiNi0.9Co0.05Mn0.05O2 (NCM955) pouch cell with an ultralean electrolyte (1.1 g Ah–1) delivers an ultrahigh energy density of 568 Wh kg–1. This solvation integration concept provides a promising guideline for the electrolyte design toward practical high-energy LMBs.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


