Ge位点预活化后GTM手性沸石催化剂的酶样对映体选择性
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
超大孔径含锗GTM手性沸石催化剂最近被证明是有用的不对称催化剂,其手性来自于其手性受限的纳米空间。然而,到目前为止,这些特殊的材料在水存在下的框架稳定性较低,并且在与1-丁醇作为测试反应的手性反式二苯乙烯氧化物开环时具有中等的催化对映选择性。在这里,我们报告了这些手性沸石催化剂在煅烧材料暴露于1-丁醇后可以很容易地稳定,提供抗水稳定性,最重要的是,促进手性活性位点的预活化,提高其对映选择性,达到前所未有的对映体过量高达88%,其中一个对映体的反应是另一个对映体的16倍。一系列的物理化学研究,包括原位傅里叶变换红外(FTIR)和x射线吸收光谱,表明框架Ge位点在与1-丁醇分子相互作用时增加了它们的配位环境,1-丁醇分子经过100°C以上的热处理后,由于缩合和脱水反应,仍然不可逆地与Ge结合,为这些材料提供了一条容易功能化的途径。这些预活化的GTM不对称催化剂的作用类似于酶,通过与Ge配合和与附近羟基形成氢键来控制手性反应物在特定方向的限制,从而获得与酶系统接近的对映选择性催化活性,但具有与多相催化剂相关的关键优势,值得注意的是,通过使用易于获取的生物碱制备催化剂的两种对映体版本的可能性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
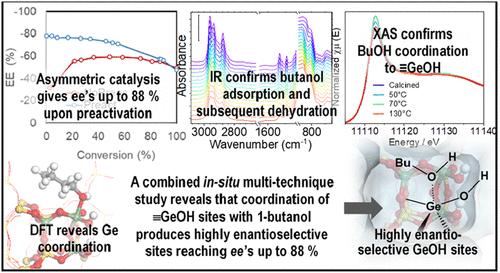
Enzyme-like Enantioselectivity in GTM Chiral Zeolite Catalysts upon Preactivation of Ge Sites
Extra-large-pore Ge-containing GTM chiral zeolite catalysts have recently proved useful asymmetric catalysts, with chirality emerging from their chiral confined nanospace. However, so far these exceptional materials have suffered from low framework stability in the presence of water and moderate catalytic enantioselectivity in the ring-opening of chiral trans-stilbene oxide with 1-butanol used as a test reaction. Here, we report that these chiral zeolite catalysts can be easily stabilized upon exposure of the calcined material to 1-butanol, providing stability against water and, most importantly, prompting a preactivation of the chiral active sites that boosts their enantioselective properties, reaching unprecedented enantiomeric excesses up to 88% where one enantiomer reacts 16 times more than the other. A range of physicochemical studies, including in situ Fourier transform infrared (FTIR) and X-ray absorption spectroscopy, indicates that framework Ge sites increase their coordination environment upon interaction with 1-butanol molecules, which after a thermal treatment above 100 °C remain irreversibly bound to Ge as a consequence of a condensation and dehydration reaction, providing a route to easily functionalize these materials. These preactivated GTM asymmetric catalysts act similarly to enzymes by controlling the confinement of the chiral reactants in particular orientations through coordination with Ge and development of H-bonds with nearby hydroxyl groups, thus attaining enantioselective catalytic activities close to those reached by enzymatic systems but with the crucial advantage associated with heterogeneous catalysts and, notably, the possibility of preparing both enantiomeric versions of the catalyst by using an easily accessible alkaloid.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


