镍催化CO2立体选择性合成二氢苯并呋喃外环烯基羧酸。
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
本文以CO2为C1源,研究了一种地球富集的镍催化碘芳系丙炔醚的Heck型环化/羧化反应。该方案提供了一系列外环烯基羧酸,具有独特的同步选择性的二氢苯并呋喃支架。克尺度的实验证明了这种转化的可扩展性,并且产品通过与丁香酚的芳构化或酯化进一步功能化,表明了潜在的应用前景。本文章由计算机程序翻译,如有差异,请以英文原文为准。
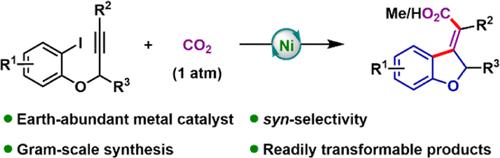
Nickel-Catalyzed Stereoselective Synthesis of Dihydrobenzofuran Exocyclic Alkenyl Carboxylic Acids from CO2
An earth-abundant nickel-catalyzed Heck type cyclization/carboxylation of iodoarene-tethered propargyl ethers using CO2 as the C1 source was developed herein. This protocol affords a series of exocyclic alkenyl carboxylic acids bearing a dihydrobenzofuran scaffold with exclusive syn-selectivity. Gram-scale experiments demonstrate the scalability of this transformation, and the products undergo further functionalization through aromatization or esterification with eugenol, indicating potential application.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


