大麻素和大麻素酸对人5-HT1A受体的结合位点和功能的硅片观察。
IF 3
4区 生物学
Q2 BIOCHEMICAL RESEARCH METHODS
引用次数: 0
摘要
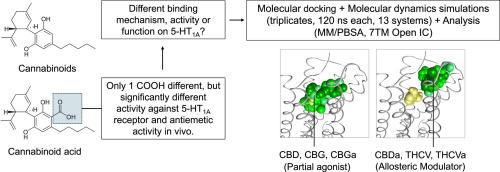
先前的研究报道,大麻素的酸性同系物,大麻二酸,在缓解呕吐方面比中性同系物大麻二酚有效约1000倍。大麻素的生物学作用可能是通过增强体树突5-HT1A受体介导的。然而,迄今为止,酸同源物增强5-HT1A活性的潜在机制仍然缺乏。为了解决这一空白,我们在人类5-HT1A受体模型中对不同对的中性和酸性大麻素进行了分子对接和分子动力学模拟。分析表明,模拟大麻素酸(大麻二酸和四氢大麻酚酸)和四氢大麻素优先结合在5-HT1A的变构位点,并且当完全激动剂R(+)-8-OH-DPAT结合在正构位点时,能够维持受体处于活性状态。重要的是,这些结果还表明,大麻二酚酸的强活性不是由于其对5-HT1A受体的强亲和力,而是由于其对5-HT1A激动剂活性的正变构调节,可能是通过阻断正构配体的出口,从而促进受体的持续激活。该研究还表明,大麻二酚和中性和酸性大麻二酚都倾向于在正位位点结合,是5-HT1A的潜在部分激动剂。总之,这些发现表明,每一种大麻素,无论是中性的还是酸性的,在其结合和功能方面都是独特的。本文章由计算机程序翻译,如有差异,请以英文原文为准。
In silico insights on the binding site and function of cannabinoids and cannabinoid acids on human 5-HT1A receptor
Previous studies reported that the acid congener of the cannabinoids, cannabidiolic acid, was approximately 1000 times more effective than the neutral congener, cannabidiol, in alleviating emesis. The biological actions of cannabinoids were proposed to be mediated by the enhancement of somatodendritic 5-HT1A receptors. However, to date, the potential mechanism that may be involved in the enhancement of the 5-HT1A activity by the acid congener is still lacking. To address this gap, molecular docking and molecular dynamics simulations were performed on different pairs of neutral and acidic cannabinoids in a human 5-HT1A receptor model. Analyses showed that simulated cannabinoid acids (cannabidiolic acid and tetrahydrocannabivarinic acid) and tetrahydrocannabivarin were preferentially bound at the allosteric site of 5-HT1A and were able to maintain the receptor in its active state when a full agonist, R(+)-8-OH-DPAT, was bound at the orthosteric site. Importantly, these results also suggest that the strong activity of cannabidiolic acid is not due to its strong affinity for the 5-HT1A receptor but its positive allosteric modulation of the agonist activity on 5-HT1A, presumably by blocking the exit of the orthosteric ligand, hence promoting continuous activation of the receptor. This study also demonstrates that cannabidiol and both neutral and acidic cannabigerol prefer binding at the orthosteric site and are potential partial agonists of 5-HT1A. In conclusion, these findings propose that every cannabinoid, regardless of whether neutral or acidic, is unique on its own in terms of its binding and function.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of molecular graphics & modelling
生物-计算机:跨学科应用
CiteScore
5.50
自引率
6.90%
发文量
216
审稿时长
35 days
期刊介绍:
The Journal of Molecular Graphics and Modelling is devoted to the publication of papers on the uses of computers in theoretical investigations of molecular structure, function, interaction, and design. The scope of the journal includes all aspects of molecular modeling and computational chemistry, including, for instance, the study of molecular shape and properties, molecular simulations, protein and polymer engineering, drug design, materials design, structure-activity and structure-property relationships, database mining, and compound library design.
As a primary research journal, JMGM seeks to bring new knowledge to the attention of our readers. As such, submissions to the journal need to not only report results, but must draw conclusions and explore implications of the work presented. Authors are strongly encouraged to bear this in mind when preparing manuscripts. Routine applications of standard modelling approaches, providing only very limited new scientific insight, will not meet our criteria for publication. Reproducibility of reported calculations is an important issue. Wherever possible, we urge authors to enhance their papers with Supplementary Data, for example, in QSAR studies machine-readable versions of molecular datasets or in the development of new force-field parameters versions of the topology and force field parameter files. Routine applications of existing methods that do not lead to genuinely new insight will not be considered.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


